Biomarker for early detection of severe hepatitis B virus as well as detection kit and application thereof
A technology for biomarkers and early detection, applied in the field of biomarkers and detection kits for early detection of severe hepatitis B virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
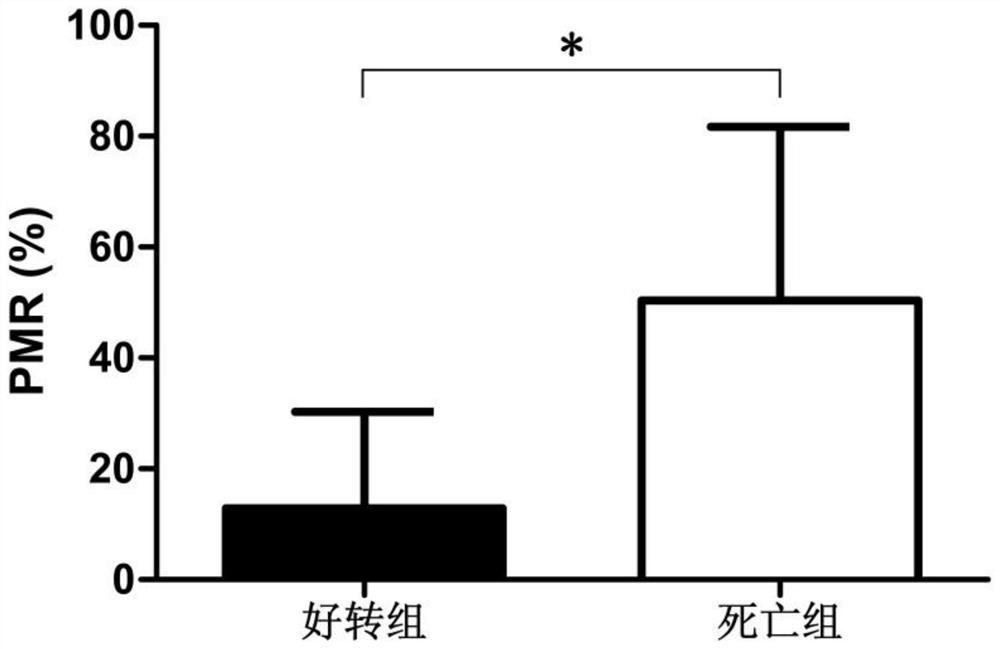
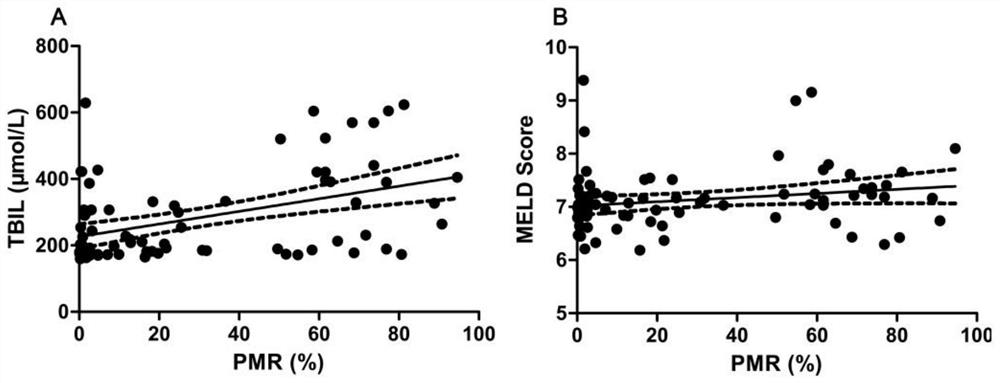
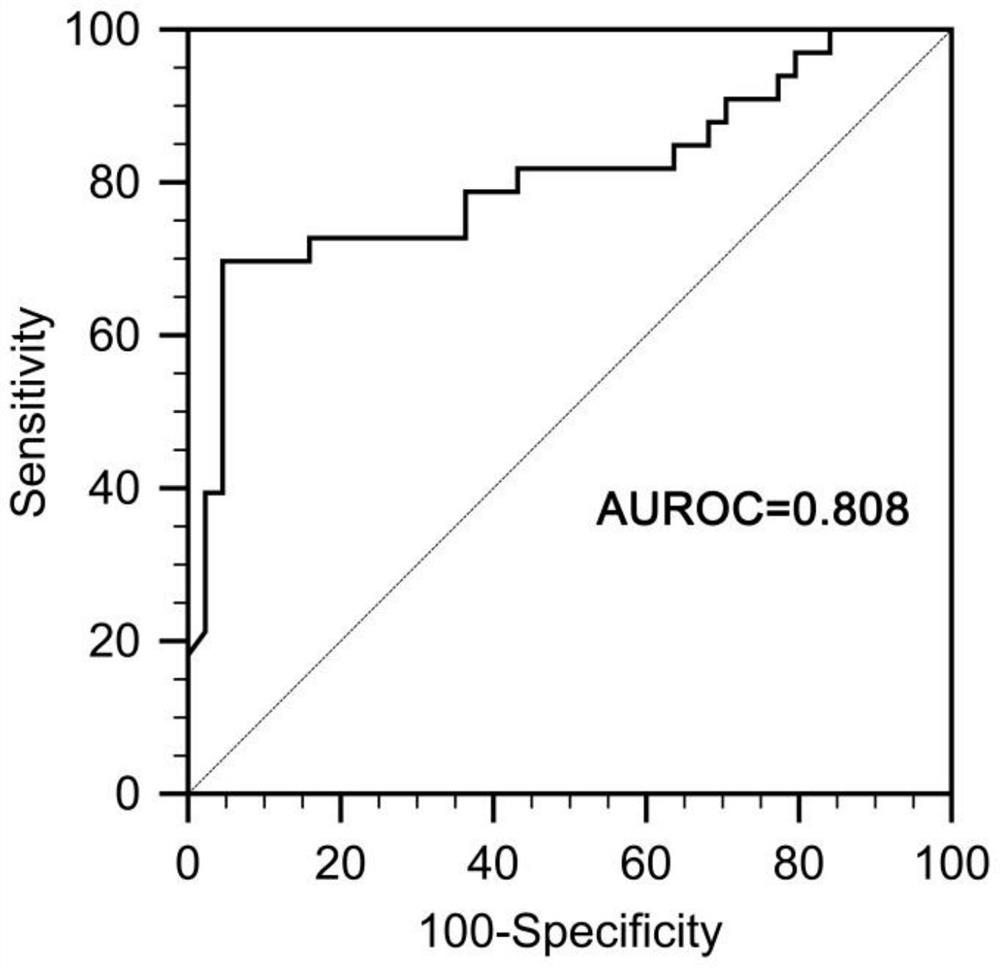
[0038] The invention mainly collects the peripheral venous blood of 77 cases of severe hepatitis B patients in Qilu Hospital of Shandong University.
[0039] Diagnostic criteria for severe hepatitis B: ① chronic hepatitis B history, HBsAg positive > 6 months; ② sudden severe jaundice with bilirubin rise ≥ 85mol / L and INR ≥ 1.5 (or PTA ≤ 40%); ③ ascites or hepatic encephalopathy.
[0040] Diagnostic criteria for chronic hepatitis B: ① HBsAg positive > 6 months; ② Serum HBV DNA > 20000 IU / mL (105 copies / mL), or 2000-20000 IU / mL (104-105 copies / mL) (common in HBeAg-negative CHB patients ); ③ Persistent or intermittent elevated ALT / AST levels; ④ Liver biopsy showed chronic liver inflammation with moderate or severe necrosis;
[0041] Exclusion criteria: ① combined with other hepatitis virus infection (hepatitis A virus, hepatitis C virus, hepatitis D virus, hepatitis E virus, etc.) and / or human immunodeficiency virus; ② suffering from other liver diseases, such as autoimmune hepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com