Lithium metal surface modification method and lithium metal battery
A lithium metal and lithium battery technology, applied in the field of material chemistry, can solve the problems of uneven deposition and lack of lithium metal, and achieve the effect of improving long-term cycle performance, avoiding the formation of growth, and achieving good and uniform deposition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A method for modifying lithium metal surfaces, comprising the steps of:
[0025] 1) Prepare electrolyte for lithium battery;
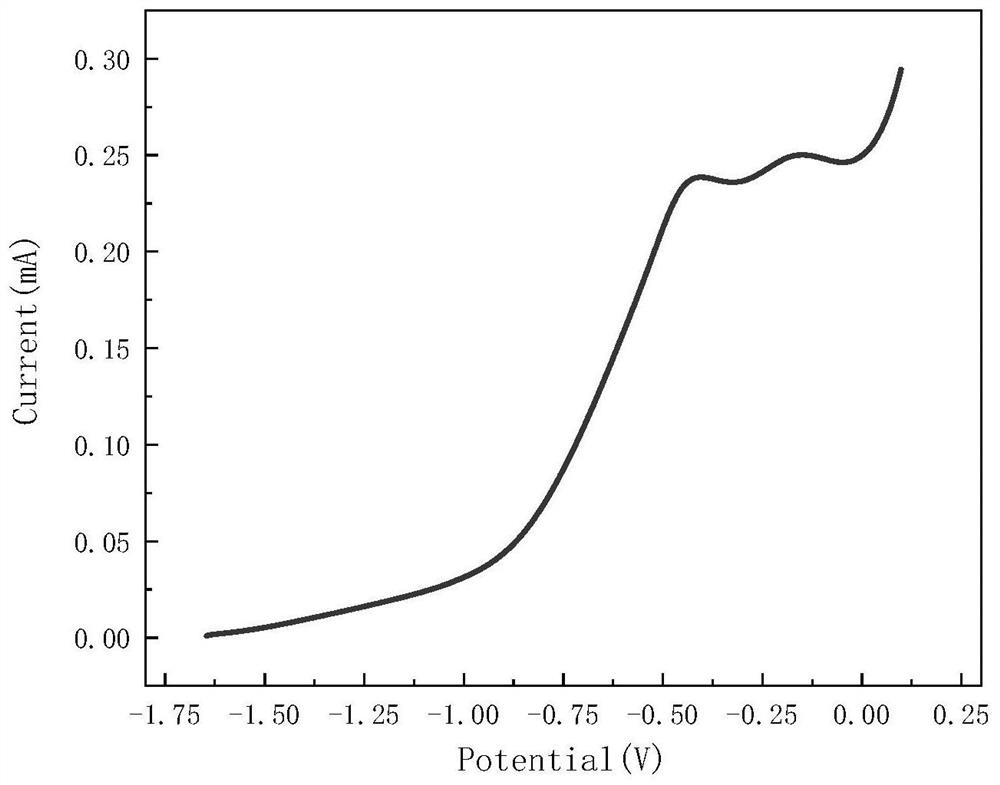
[0026] 2) Use the electrolyte in step 1) for a two-electrode system to measure the polarization curve of lithium metal in the electrolyte, and obtain the voltage parameters of the optimal passivation region of lithium metal according to the polarization curve;
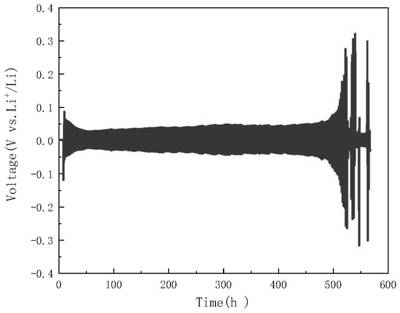
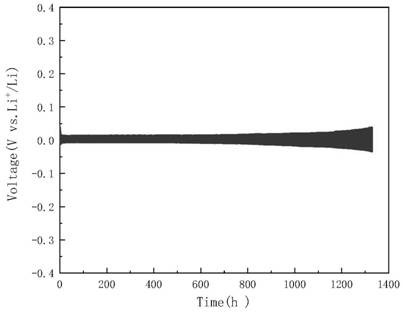
[0027] 3) Assemble a lithium metal symmetric battery, use lithium metal as the positive and negative electrodes, and add the electrolyte prepared in step 1) to wet the diaphragm, seal the assembled lithium metal symmetric battery, and then use the voltage parameters obtained in step 2) The constant potential charge treatment is carried out on the lithium metal symmetric battery to obtain the lithium metal after surface modification.
[0028] In this example, the preparation of the lithium battery electrolyte in step 1) specifically includes: weighing a certain amount of lithium bromide ...
Embodiment 2
[0033] Weigh 1 mmol (0.0868 g) lithium bromide (LiBr) and 20 mmol (1.3788 g) lithium nitrate (LiNO3 ) into 20 mL of tetraethylene glycol dimethyl ether (TEGDME), then sealed and stirred for 2 h to obtain a clear electrolyte.
[0034] Then the prepared electrolyte solution was added to a 50mL double-hole sealed electrolytic cell. The working electrode used a lithium sheet (d=15.6 mm), the auxiliary electrode and the reference electrode were made of stainless steel sheets, and the LSV ( Linear Sweep Voltammetry, linear sweep voltammetry) program to measure the polarization curve of lithium metal in the above electrolyte, the test parameters are set as: Init E (initial potential, V) = open circuit voltage, Final E (end potential, V) = 0.1, ScanRate (scanning speed, V / s)=0.005, Sample Interval (sampling interval, V)=0.001, Quiet Time (quiet time, sec)=2, Sensitivity (sensitivity, A / V)=1.0×10 -3 , to obtain the polarization curve of lithium metal in the electrolyte.
[0035] Use t...
Embodiment 3
[0037] Weigh 1.6 mmol (0.1388 g) lithium bromide (LiBr) and 20 mmol (1.3788 g) lithium nitrate (LiNO 3 ) into 20 mL of tetraethylene glycol dimethyl ether (TEGDME), then sealed and stirred for 2 h to obtain a clear electrolyte.
[0038] Then the prepared electrolyte solution was added to a 50mL double-hole sealed electrolytic cell. The working electrode used a lithium sheet (d=15.6 mm), the auxiliary electrode and the reference electrode were made of stainless steel sheets, and the LSV ( Linear Sweep Voltammetry, linear sweep voltammetry) program to measure the polarization curve of lithium metal in the above electrolyte, the test parameters are set as: Init E (initial potential, V) = open circuit voltage, Final E (end potential, V) = 0.1, ScanRate (scanning speed, V / s)=0.005, Sample Interval (sampling interval, V)=0.001, Quiet Time (quiet time, sec)=2, Sensitivity (sensitivity, A / V)=1.0×10 -3 , the polarization curve of lithium metal in the electrolyte is obtained as figure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com