Preparation method of lithium difluoro(oxalato)borate
A technology for preparing lithium difluorooxalate borate and a device, which is applied in the direction of chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, evaporation, separation and crystallization, etc. Lithium oxalate borate cost, long reaction time and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
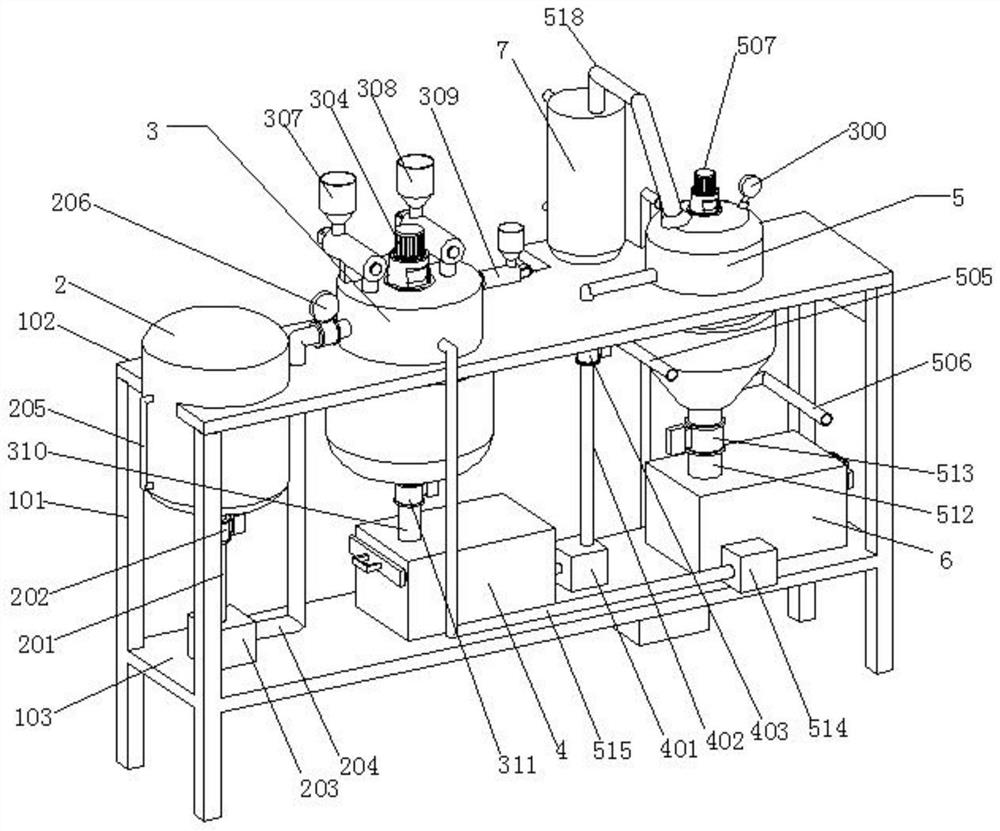
Image
Examples
Embodiment 1
[0048] A preparation method of lithium difluorooxalate borate, comprising the steps of:
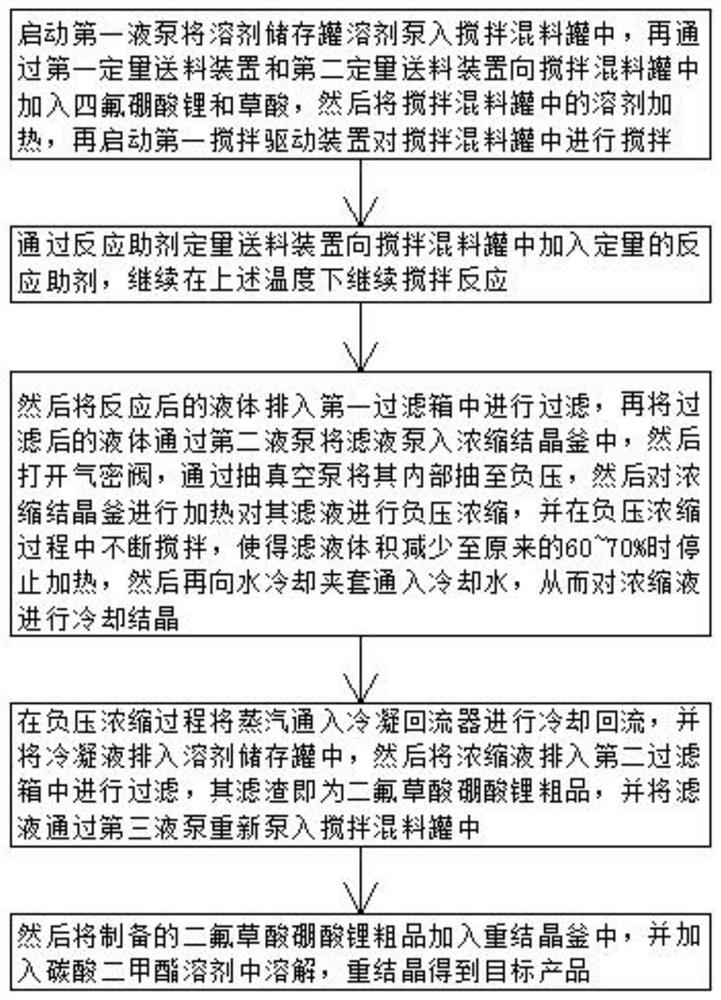
[0049] 1) Start the first liquid pump to pump the solvent from the solvent storage tank into the stirring and mixing tank, then add lithium tetrafluoroborate and oxalic acid to the stirring and mixing tank through the first quantitative feeding device and the second quantitative feeding device, and then put The solvent in the stirring mixing tank was heated to 55° C., and then the first stirring driving device was started to stir in the stirring mixing tank for 8 minutes, wherein the stirring speed of the first stirring driving device was 360 r / min.
[0050] 2) Add a certain amount of aluminum trichloride as a reaction aid to the stirring mixing tank through the reaction aid quantitative feeding device, and continue to stir and react at a temperature of 55° C. for 2.5 hours; wherein, lithium tetrafluorophosphate, acetonitrile solution and The molar mass ratio of reaction aids is 12:14:3. ...
Embodiment 2
[0055] A preparation method of lithium difluorooxalate borate, comprising the steps of:
[0056] 1) Start the first liquid pump to pump the solvent from the solvent storage tank into the stirring and mixing tank, then add lithium tetrafluoroborate and oxalic acid to the stirring and mixing tank through the first quantitative feeding device and the second quantitative feeding device, and then put The solvent in the stirring mixing tank was heated to 60° C., and then the first stirring driving device was started to stir in the stirring mixing tank for 6 minutes, wherein the stirring speed of the first stirring driving device was 400 r / min.
[0057] 2) Add a certain amount of silicon tetrachloride as a reaction aid to the stirring mixing tank through the reaction aid quantitative feeding device, and continue to stir and react at a temperature of 55° C. for 2.6 hours; wherein, lithium tetrafluorophosphate, acetonitrile solution and The molar mass ratio of the reaction auxiliary ag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com