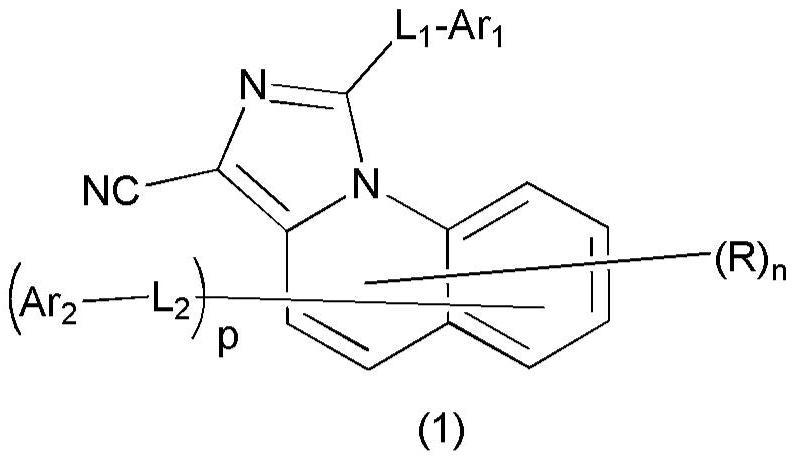
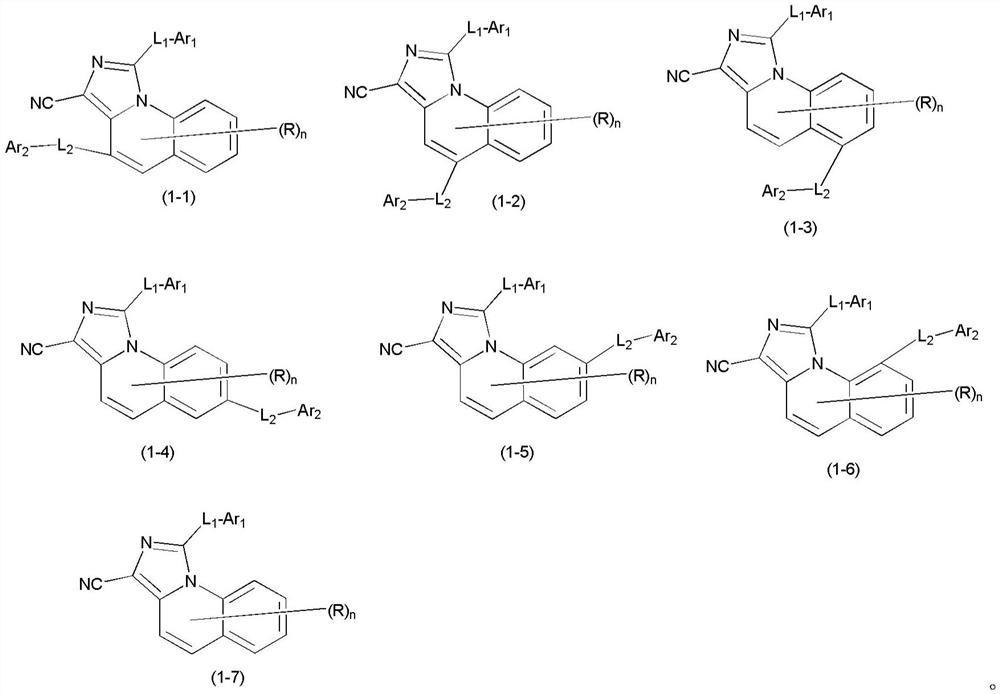
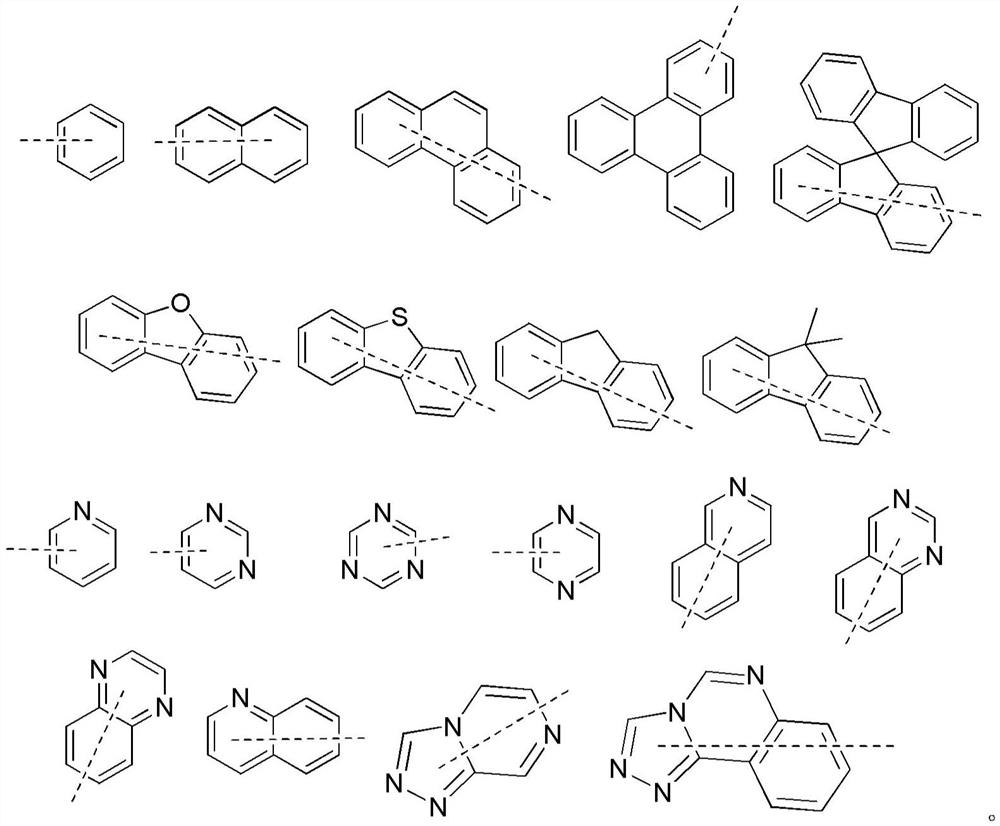
Organic compound and organic electroluminescent device containing the same
An organic compound, unsubstituted technology, applied in organic chemistry, electric solid-state devices, luminescent materials, etc., to achieve good planar conjugation, good electron injection and migration performance, and improve mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0051]Synthesis Example 1: Synthesis of Compound C2
[0052]
[0053](1) Preparation of Compound 1-1
[0054]2-methylquinoline (14.3 g, 0.1 mol, 1 eq), 4-bromine (55.5 g, 0.3 mol, 3 eq), TMSCN (30g, 0.3 mol, 3eq), NH4I (2.9g, 0.02mol, 0.2eq), pH2PO2H (21.8g, 0.1 mol, 1 eq) and NH4BF4(10.5 g, 0.1 mol, 1 eq) was added to a three-mouth flask containing a platinum electrode containing DMA (1L). Under power catalysis (j = 10mA / cm2The oil bath is heated to 90 ° C for 20 hours, and the TLC monitored reaction is completed. The reaction liquid was cooled to room temperature, and the solvent was spurly evaporated under reduced pressure. 1.5 L of water was added and extracted with ethyl acetate (1 L * 3). The ethyl acetate phase was combined, dried over anhydrous sodium sulfate, filtration, and evaporated. Ethyl acetate, the resulting crude product column layer was purified to the intermediate 1-1 (27.2 g, yield 80%).
[0055](2) Preparation of Compound C2
[0056]Compound 1-1 (6.2 g, 18 mmol), Compound 2-...
Synthetic example 2
[0057]Synthesis 2: Synthesis of Compound C22
[0058]
[0059](1) Preparation of Compound 2-1
[0060]2-methylquinoline (14.3 g, 0.1 mol, 1 eq), 3-chlorobenzylamine (42.3 g, 0.3 mol, 3eq), TMSCN (30g, 0.3 mol, 3eq), NH4I (2.9g, 0.02mol, 0.2eq), pH2PO2H (21.8g, 0.1 mol, 1 eq) and NH4BF4(10.5 g, 0.1 mol, 1 eq) was added to a three-mouth flask containing a platinum electrode containing DMA (1L). Under power catalysis (j = 10mA / cm2The oil bath is heated to 90 ° C for 22 hours, and the TLC monitored reaction is completed. The reaction liquid was cooled to room temperature, and the solvent was spurly evaporated under reduced pressure. 1.5 L of water was added and extracted with ethyl acetate (1 L * 3). The ethyl acetate phase was combined, dried over anhydrous sodium sulfate, and evaporated with EtOAc (EtOAc) EtOAc.
[0061](2) Preparation of Compound 2-2
[0062]Compounds 2-1 (15.5 g, 50 mmol), ferric acid-ravate (19 g, 75 mmol) and potassium acetate (14.7 g, 150 mmol) were added to a flask containin...
Synthetic example 3
[0065]Synthesis Example 3: Synthesis of Compound C41
[0066]
[0067](1) Preparation of Compound 3-1
[0068]Compound 4- (4-bromophenyl) benzengnitrile (25.7 g, 0.1 mol), 3-methyl-4-chlorine-phenyl boric acid (107 g, 0.4 mol), potassium carbonate (17 g, 0.1 mol), Pd (PPH3)4(1155 mg, 1 mmol) was added to a flask containing toluene / ethanol / water 400 ml / 100 ml / 100 ml, and the replacement of nitrogen was reacted for 4 hours in a nitrogen atmosphere, and the TLC showed the reaction. It was cooled to room temperature, separation, and aqueous phase extraction was extracted with ethyl acetate, combined with an organic phase, sodium anhydrous sodium sulfate, and purified compounds 3-1 (25 g, 83%).
[0069](2) Preparation of Compound 3-2
[0070]Compounds 3-1 (15.2 g, 50 mmol), boronic acid-ravata (19 g, 75 mmol) and potassium acetate (14.7 g, 150 mmol) were added to a flask containing 1,4-dioxane (300 mL), room temperature After stirring, the nitrogen was stirred and palladium (224 mg, 1 mmol), SP...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electric potential / voltage | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com