Method for preparing o-fluorophenol from stable triazene intermediate
A technology for o-fluorophenol and triazene, applied in the field of preparing o-fluorophenol, can solve the problems of difficulty in generating economic benefits, large potential safety hazards, poor stability, etc., and achieves avoiding coupling side reactions, high safety, and improving yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
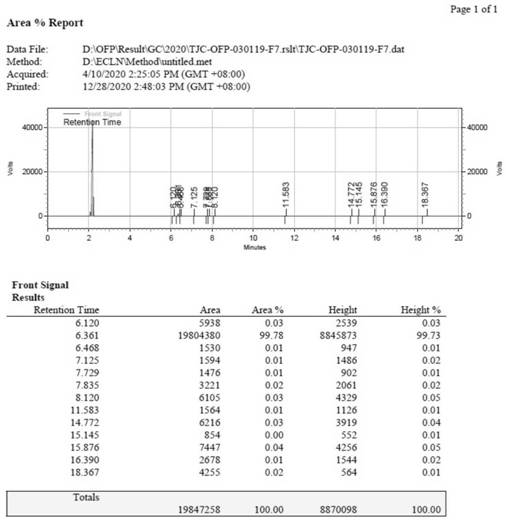
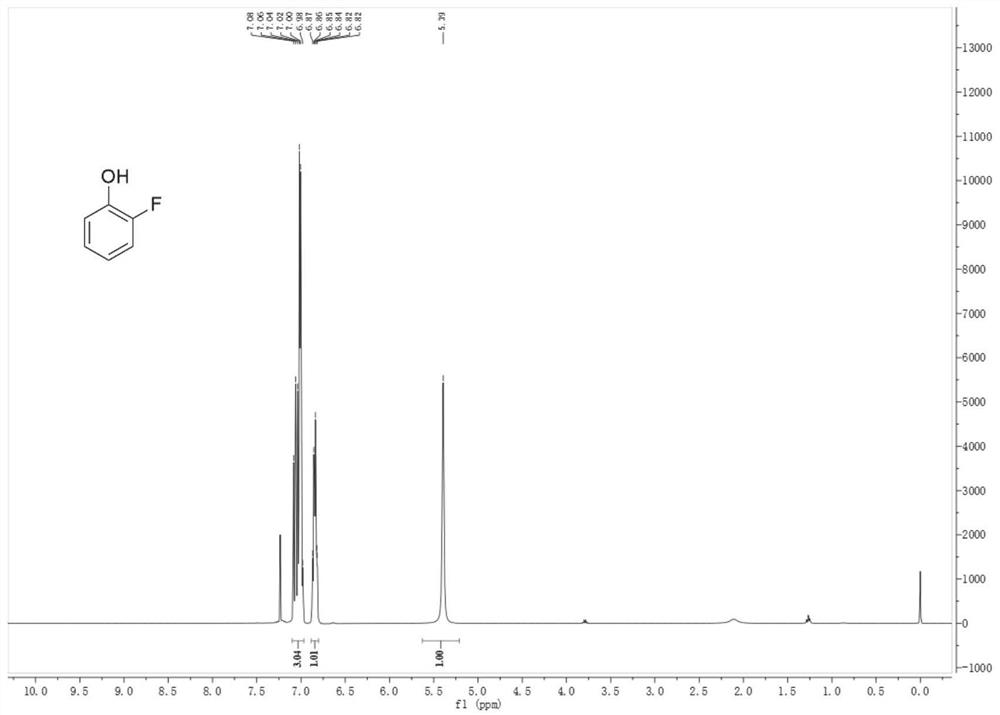
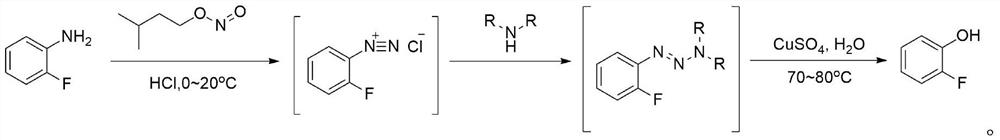
[0035] Put 3L of water into the 10L four-necked bottle 1, then slowly add 978.5g of 36% hydrochloric acid dropwise, and add dropwise while stirring. After the dropwise addition, the temperature of the prepared diluted hydrochloric acid is 40-50°C. Then, the temperature was lowered to 10-15° C., and 500 g of o-fluoroaniline was quickly dropped into bottle 1 to form a salt. Then, continue to lower the temperature to 0-5°C and add 606.1 g of isoamyl nitrite dropwise for 1 hour. After the dropwise addition is completed, keep warm at 0-5°C and react for 0.5h, then take a sample, and use starch potassium iodide test paper to detect that it first turns purple-black, and then turns blue. Then, dropwise add a solution prepared by 510.2 g of morpholine and 500 mL of acetonitrile into the reaction bottle 1 at 0-5° C., after the addition is completed, keep warm at 0-5° C. for 1 hour. Put 2L of water and 561.8g of copper sulfate pentahydrate into the 10L four-necked bottle 2 equipped with...
Embodiment 2
[0037] Put 4L of water into the 10L four-necked bottle 1, then slowly add 1293.6g of 36% hydrochloric acid dropwise, and add dropwise while stirring. After the dropwise addition, the temperature of the prepared dilute hydrochloric acid is 40-50°C. Then, the temperature was lowered to 10-15° C., and 500 g of o-fluoroaniline was quickly dropped into bottle 1 to form a salt. Then, continue to lower the temperature to 0-5°C and add 632.5 g of isoamyl nitrite dropwise for 1 hour. After the dropwise addition is completed, keep warm at 0-5°C and react for 0.5h, then take a sample, and use starch potassium iodide test paper to detect that it first turns purple-black, and then turns blue. Then, dropwise add a solution prepared by 587.8 g of morpholine and 600 mL of acetonitrile into the reaction bottle 1 at 0-5° C., after the addition is completed, keep warm at 0-5° C. for 1 hour. Put 2.5L of water and 702.2g of copper sulfate pentahydrate into the 10L four-neck bottle 2 equipped with...
Embodiment 3
[0039] Put 3.5L of water into the 10L four-necked bottle 1, then slowly add 1140.3g of 36% hydrochloric acid dropwise, and add dropwise while stirring. After the dropwise addition, the temperature of the prepared dilute hydrochloric acid is 40-50°C. Then, the temperature was lowered to 10-15° C., and 500 g of o-fluoroaniline was quickly dropped into bottle 1 to form a salt. Then, continue to lower the temperature to 0-5°C and add 613.2 g of isoamyl nitrite dropwise for 1 hour. After the dropwise addition is completed, keep warm at 0-5°C and react for 0.5h, then take a sample, and use starch potassium iodide test paper to detect that it first turns purple-black, and then turns blue. Then, dropwise add the solution prepared by 498.6 g of hexahydropyridine and 500 mL of acetonitrile into the reaction bottle 1 at 0-5° C., after the addition is completed, keep the temperature at 0-5° C. for 1 hour. Put 2L of water and 561.8g of copper sulfate pentahydrate into the 10L four-necked ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com