Preparation method of sofosbuvir impurity
A technology for sofosbuvir and impurities, which is applied in the field of preparation of sofosbuvir impurities and achieves the effects of mild reaction conditions, simple operation and good impurity purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
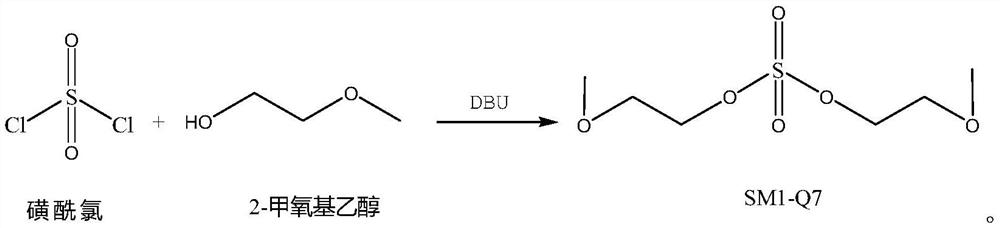
[0038] Add 42g of 2-methoxyethanol, 85g of DBU, and 150g of DMF in sequence in a 250mL reaction bottle, stir rapidly at room temperature for 15 minutes, add 30g of sulfonyl chloride dropwise, control the reaction temperature below 40°C, and add dropwise for 30 minutes. Finally, react at about 40°C for 3 hours, then raise the temperature to about 60°C for 3 hours. After the reaction is completed, concentrate under reduced pressure below 95°C until there is no distillate, add 150g of dichloromethane and 30g of water, stir and dissolve for 30 minutes, separate the layers, wash the organic layer with water twice, and then separate the layers, and concentrate the organic layer under reduced pressure below 50°C. Layer until there is no fraction, and the residue is obtained, which is the crude product of SM1-Q7. The crude product was separated by silica gel column flash chromatography (eluent: ethyl acetate / n-hexane=8 / 1), and the fractions were collected and concentrated to obtain ab...
Embodiment 2
[0040]Add 37g of 2-methoxyethanol, 37g of DBU, and 100g of tetrahydrofuran to a 250mL reaction flask in sequence, stir rapidly at room temperature for 15 minutes, add 30g of sulfuryl chloride dropwise, control the reaction temperature below 40°C, and add dropwise for 30 minutes. After completion, react at about 40°C for 3 hours, then raise the temperature to about 50°C for 3 hours. After the reaction is complete, concentrate under reduced pressure below 50°C until there is no distillate, add 150g of dichloromethane and 30g of water, stir and dissolve for 40 minutes, separate the layers, wash the organic layer with water twice, and then separate the layers, and concentrate the organic layer under reduced pressure below 50°C. Layer until there is no fraction, and the residue is obtained, which is the crude product of SM1-Q7. The crude product was separated by silica gel column flash chromatography (eluent: ethyl acetate / n-hexane=8 / 1), and the fractions were collected and concent...
Embodiment 3
[0042] Add 37g of 2-methoxyethanol, 74g of DBU, and 150g of acetonitrile to a 500mL reaction flask in sequence, stir rapidly at room temperature for 15 minutes, add 30g of sulfonyl chloride dropwise, control the reaction temperature below 40°C, and add dropwise for 30 minutes. After completion, react at about 40°C for 3 hours, then raise the temperature to about 50°C for 3 hours. Post-treatment was carried out according to Example 2 (eluent: ethyl acetate / n-hexane=8 / 1), and about 36 g of the colorless liquid target SM1-Q7 was obtained with a purity of 98.9%.
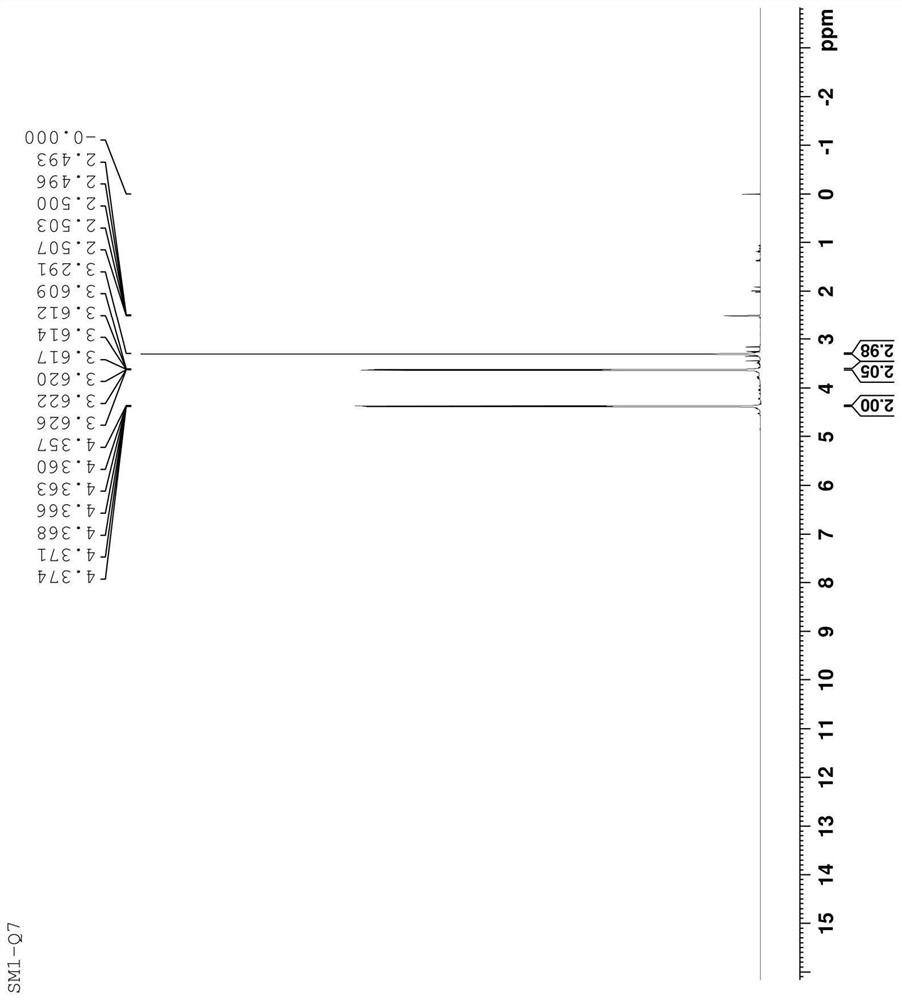
[0043] The structure of the prepared compound was confirmed by hydrogen spectroscopy, and it was confirmed to be the impurity SM1-Q7. The analysis is as follows: 1 HNMR (500MHz, d-DMSO); δ: 3.291 (S, 3H), equivalent to 6H, in line with the number of protons of two symmetrical methyl groups in the molecule; δ: 3.609-3.626 (m, 2H), equivalent to 4H, in line with The number of protons of the two symmetrical methylene group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com