Method for preparing oxazoline insecticide arforaging intermediate
A technology of intermediates and pesticides, applied in the field of pharmaceuticals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
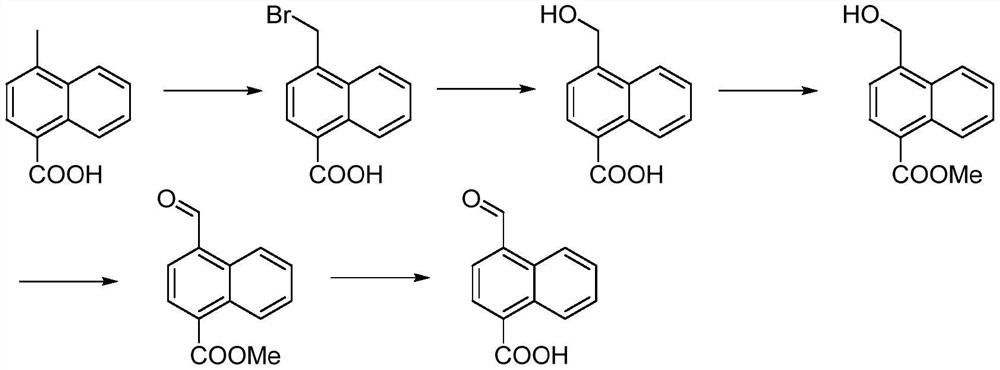
[0059] Halogenation reaction
[0060] Preparation of 4-(bromomethyl)-naphthalene-1-carboxylic acid
[0061]
[0062]1) In a 500ml round bottom flask, add acetonitrile 200ml, 4-methyl-naphthalene-1-carboxylic acid 26g (0.14mol), 1,3-dibromo-5,5-dimethylhydantoin 60.04g ( 0.21mol) and then the temperature rose to 50-60°C, and the reaction was continued for 3 hours;
[0063] 2) Concentrate under reduced pressure, extract with dichloromethane, wash the organic layer successively with water and 15% saline, dry over anhydrous sodium sulfate, filter, and distill off the solvent under reduced pressure at 45°C to obtain 4-(methyl bromide Base) naphthalene-1-carboxylic acid 32.85g, yield 88.5%.
[0064] MS (m / z): [M-H] + = 264.32. 1 H NMR (400MHz, DMSO-D 6 ): δ5.38 (s, 2H), 7.75 (m, 3H), 8.11 (d, 1H), 8.44 (d, 1H), 8.73 (d, 1H), 13.41 (brd s, 1H).
[0065] Hydrolysis reaction
[0066] Preparation of 4-(hydroxymethyl)-naphthalene-1-carboxylic acid
[0067]
[0068] 3) In a ...
Embodiment 2
[0080] Halogenation reaction
[0081] Preparation of methyl 4-(bromomethyl)-1-naphthoate
[0082]
[0083] 1) In a 500ml round bottom flask, add 240ml of acetonitrile, 14.02g (0.07mol) of methyl 4-methyl-1-naphthoate, and 25.73g of 1,3-dibromo-5,5-dimethylhydantoin (0.09mol) after the temperature rose to 50 ~ 60 ℃, continue to react for 3 hours;
[0084] 2) Concentrate under reduced pressure, extract with dichloromethane, wash the organic layer successively with water and 15% saline, dry over anhydrous sodium sulfate, filter, and distill off the solvent under reduced pressure at 45°C to obtain 4-(methyl bromide Base)-1-naphthoic acid methyl ester 17.45g, yield 89.3%.
[0085] MS (m / z): [M-H] + =278.33. 1 H NMR(400MHz, CDCl3): δ:4.14(s,3H),4.88(s,2H),7.76(d,1H),7.57-7.69(m,2H),8.36(d,1H),8.15-8.18 (m,1H),8.89-8.95(m,1H).
[0086] Hydrolysis reaction
[0087] Preparation of 4-(hydroxymethyl)-naphthalene-1-carboxylic acid
[0088]
[0089] 3) In a 500ml round bottom...
Embodiment 3
[0101] Halogenation reaction
[0102] Preparation of 4-(bromomethyl)-naphthalene-1-carboxylic acid
[0103]
[0104] 1) In a 500ml round-bottomed flask, add 310ml of dichloromethane, 20.5g (0.11mol) of 4-methyl-naphthalene-1-carboxylic acid, and 26g (0.16mol) of bromine in sequence, and then the temperature rises to 50-60°C. Continue to react for 2 hours;
[0105] 2) The organic phase layer was washed with water and 15% saline successively, dried over anhydrous sodium sulfate, filtered, and the solution was distilled off under reduced pressure at 45°C to obtain 4-(bromomethyl)naphthalene-1-carboxylic acid 25.5 g, yield 87.5%
[0106] MS (m / z): [M-H] + = 264.32. 1 H NMR (400MHz, DMSO-D 6 ): δ5.38(s,2H),7.75(m,3H),8.11(d,1H),8.44(d,1H),8.73(d,1H),13.41(brds,1H).
[0107] Hydrolysis reaction
[0108] Preparation of 4-(hydroxymethyl)-naphthalene-1-carboxylic acid
[0109]
[0110] 3) In a 500ml round bottom flask, add 300ml of water, 13.3g (0.05mol) of 4-(bromomethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com