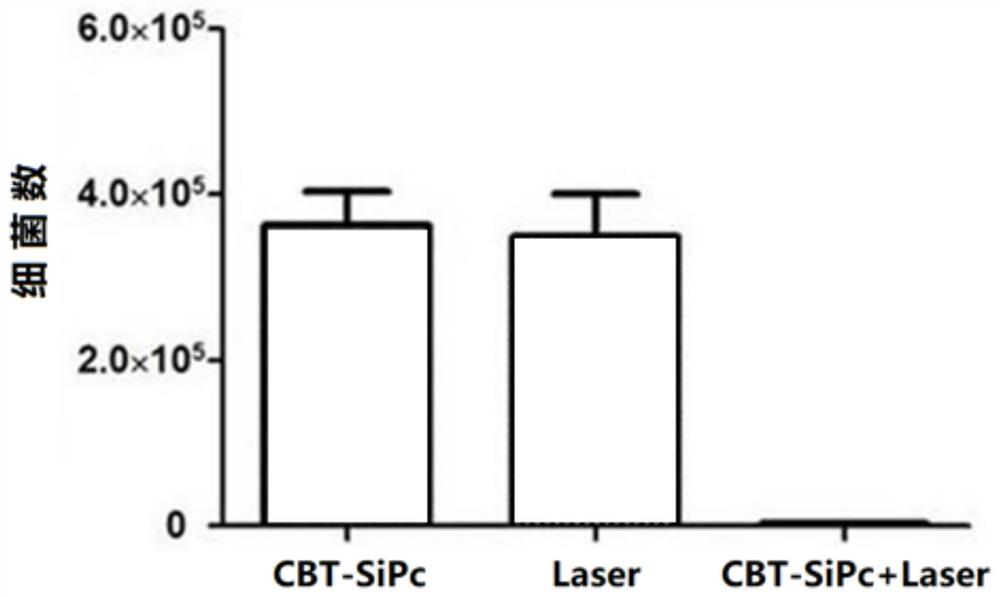
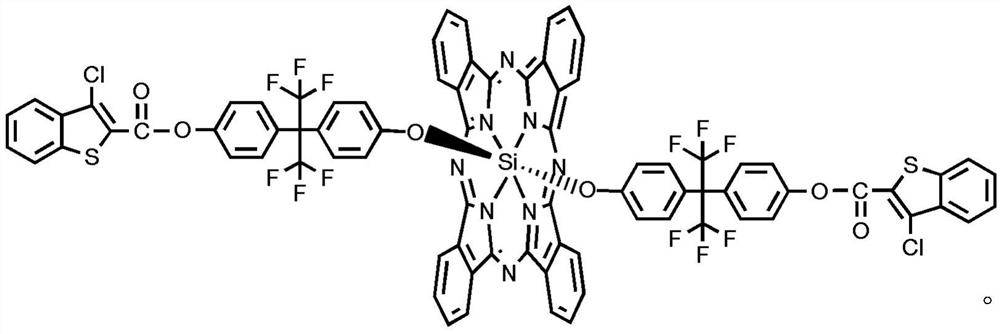
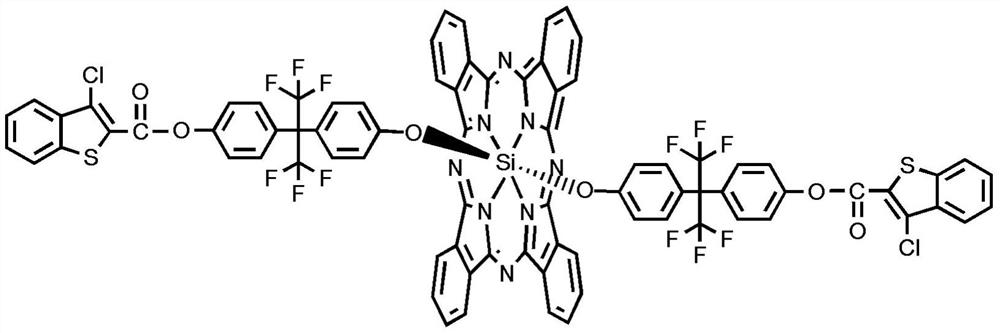
Preparation method of di-((3-chlorobenzothiophene-2-ester) hexafluorophenoxy) axially substituted silicon phthalocyanine
A technology of hexafluorophenoxy and chlorobenzothiophene is applied in chemical instruments and methods, medical preparations containing active ingredients, pharmaceutical formulations, etc., to achieve the effects of increasing penetrating ability, increasing solubility, and increasing permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) dichlorosilicon phthalocyanine (abbreviated as SiPcCl in the present invention 2 )Synthesis
[0024] Add 1,3-diiminoisoindoline (7.28g, 50.15mmol), silicon tetrachloride 8.3mL and quinoline 83mL respectively into a three-necked flask, stir and reflux at 220°C for 30min, cool to room temperature, and It was poured into 500mL of methanol solution, stirred and left for about 1 hour, filtered, and the filter residue was washed with 35mL of acetone, methanol, dichloromethane, methanol and other solvents. After drying, 3.6759g of a purple solid was obtained, with a yield of 48.62%.
[0025] 2) Synthesis of 3-chloro-benzothiophene-2-ester-based hexafluorophenol (abbreviated as CBT-OH in the present invention)
[0026] 0.425 g (2 mmol) of 3-chlorobenzothiophene-2-carboxylic acid, 0.67 g (2 mmol) of hexafluorobisphenol A in 70 mg (0.6 mmol) of 4-diaminopyridine, 1 -Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (0.5g, 2.6mmol) and dichloromethane (40mL), stirred at...
Embodiment 2
[0032] The specific steps are the same as in Example 1: in the process, the amount of 3-chlorobenzothiophene-2-carboxylic acid is 0.850 g (4 mmol), the amount of hexafluorobisphenol A is 0.134 g (4 mmol), and the reaction temperature is 90 ° C. Other reaction conditions were the same, and 0.22 g of ginger green liquid product was obtained with a yield of 21.2%.
[0033] The specific steps are the same as in Example 1: in the process, the amount of 3-chloro-benzothiophene-2-ester-based hexafluorophenol is 0.232g (0.4mmol), the amount of dichlorosilicon phthalocyanine is 0.12g (0.2mmol), and no water Potassium carbonate changed to K 2 CO 3 1.2g (9mmol), the reaction temperature was changed to 130°C. Other reaction conditions were the same, and 0.82 g of white fluffy product was obtained, yield: 44%.
Embodiment 3
[0035] The specific steps are the same as in Example 1: in the process, the dosage of 3-chlorobenzothiophene-2-carboxylic acid is 1.7g (8mmol), and the dosage of hexafluorobisphenol A is 0.134g (4mmol). The reaction temperature is changed to 110°C. Other reaction conditions were the same, and 0.25 g of the ginger green liquid product was obtained with a yield of 23%.
[0036] The specific steps are the same as in Example 1: in the process, the consumption of 3-chloro-benzothiophene-2-ester base hexafluorophenol is 0.464g (0.8mmol), and the consumption of dichlorosilicon phthalocyanine is 0.12g (0.2mmol). The temperature was changed to 150°C. Other reaction conditions were the same, and 0.90 g of white fluffy product was obtained, yield: 46.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com