A class of oral gsnor inhibitors and their pharmaceutical use
A technology of inhibitors and drugs, applied in the direction of drug combinations, antineoplastic drugs, pharmaceutical formulations, etc., can solve the problem of reduced activity, achieve the effects of increased permeability, promotion of angiogenesis and blood flow recovery, and long half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
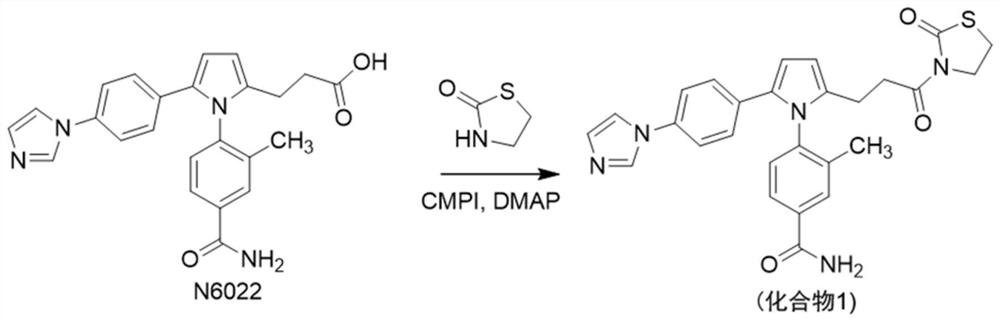
[0024] Example 1: 4-(2-(4-(1H-imidazol-1-yl)phenyl)-5-(3-oxo-3-(2-oxothiazolidin-3-yl)propyl)- Synthesis of 1H-pyrrol-1-yl)-3-methylbenzamide (target compound 1), as figure 1 shown.
[0025] Experimental operation:
[0026] In a 250 ml single-necked flask, successively put 3-(5-(4-(1H-imidazol-1-yl)phenyl)-1-(4-carbamoyl-2-methylphenyl)-1H-pyrrole -2-yl)propionic acid (N6022, 500mg, 1.21mmol), 2-chloro-1-picoline iodide (CMPI, 370mg, 1.45mmol), dimethylaminopyridine (DMAP, 10mg, 0.06mmol), thiazole Add alkanone (250mg, 2.41mmol), triethylamine (TEA, 490.2mg, 4.84mmol), add dichloromethane (15ml), and react at room temperature for 8 hours. Suction filtration, take the filtrate, adjust the pH to 7 with dilute hydrochloric acid, add dichloromethane (15ml) and water (15ml) for extraction, combine the organic phases, spin-steam to make sand, and separate by column chromatography (dichloromethane:methanol=25:1 ) to obtain 400 mg of a beige crude product, and recrystallized from ...
Embodiment 2
[0028] Example 2: In vitro activity research of target compound 1 on asthma and cystic fibrosis (with N6022, N6022-1, N6022-2 as controls)
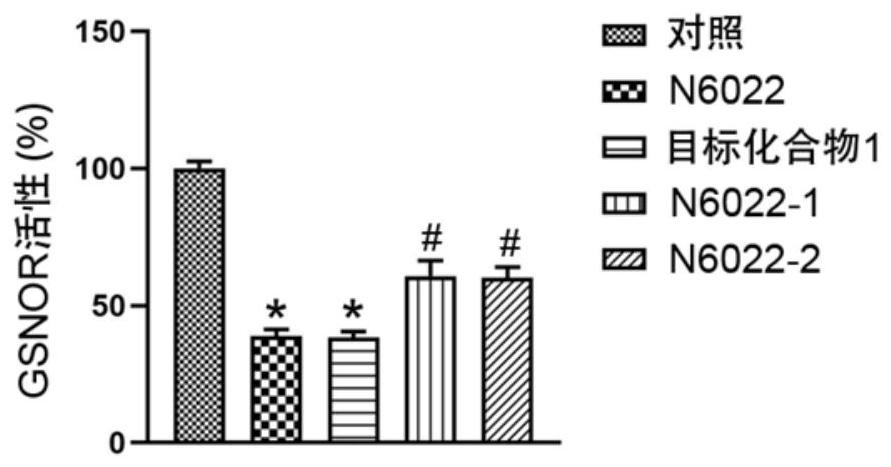
[0029]GSNOR plays a key regulatory role in asthma and cystic fibrosis; the activity of GSNOR in the lungs of asthmatic patients increases significantly, and GSNOR allele mutations lead to a significant increase in the probability of children suffering from asthma; at the same time; the respiratory tract of cystic fibrosis patients The activity of GSNOR increased significantly, and the level of its catalytic product GSNO decreased significantly; therefore, in order to explore the protective effect of target compound 1 in asthma and cystic fibrosis, we tested the effect of target compound 1, N6022, N6022-1 and N6022-2 in vitro Inhibitory effect of GSNOR activity. Such as figure 2 As shown, the in vitro activity study of the target compound 1 on asthma and cystic fibrosis. Human umbilical vein endothelial cells (HUVECs) were treated with ...
Embodiment 3
[0031] Example 3: Study on the In Vitro Activity of Target Compound 1 on Diabetic Vascular Complications (with N6022, N6022-1, and N6022-2 as Controls)
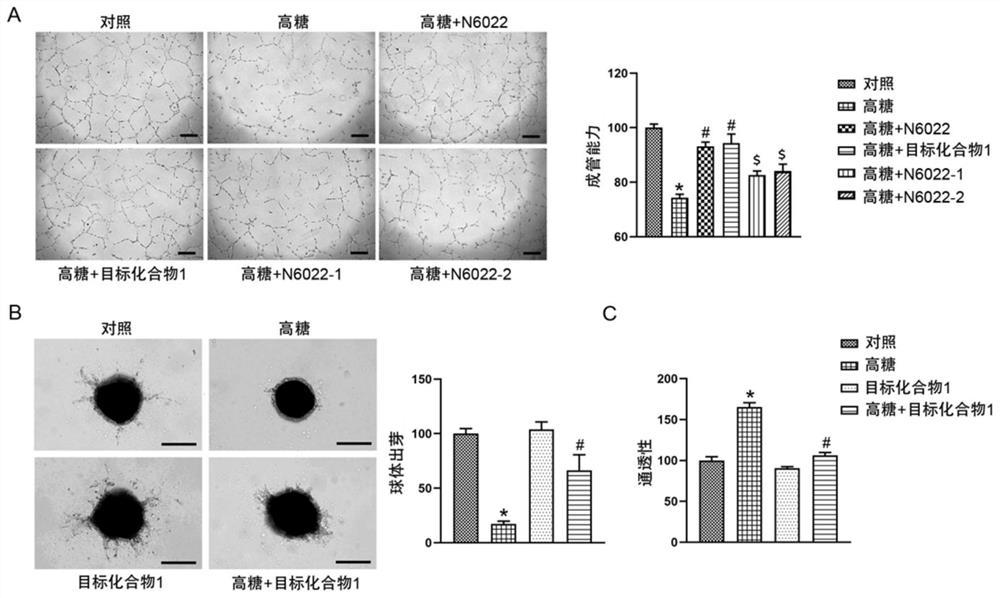
[0032] It is known that in diabetic vascular complications, decreased angiogenesis and increased permeability of vascular endothelial cells are one of the risk factors for local edema, ischemia followed by ulcer and amputation. In order to study the therapeutic effect of target compound 1 on diabetic vascular complications, we administered high glucose (30mM) stimulation to human umbilical vein endothelial cells (HUVECs) at the cellular level, and simultaneously administered target compound 1, N6022, N6022-1 and N6022-2 (10nM) for 24 hours, and the angiogenesis ability of endothelial cells was detected by endothelial cell tube formation assay. Such as image 3 As shown, the in vitro activity study of the target compound 1 on diabetic vascular complications. Human umbilical vein endothelial cells (HUVECs) were given high glu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com