Novel magnesium-serinate compound and use thereof
A compound, serine technology, applied in the direction of magnesium organic compounds, heavy metal compounds active ingredients, applications, etc., can solve the problem of insufficient amount and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
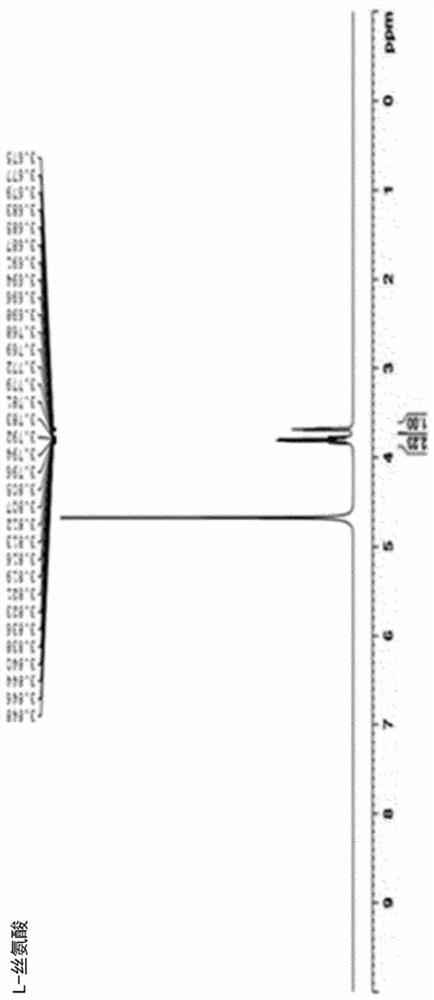
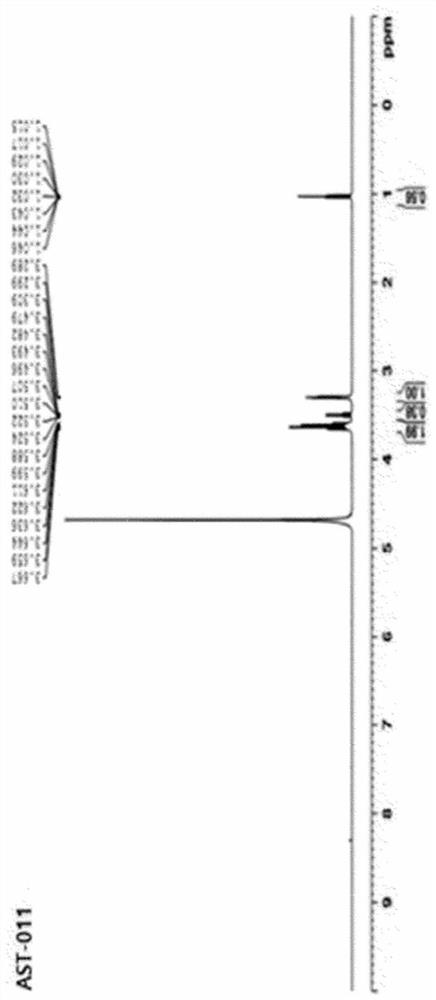
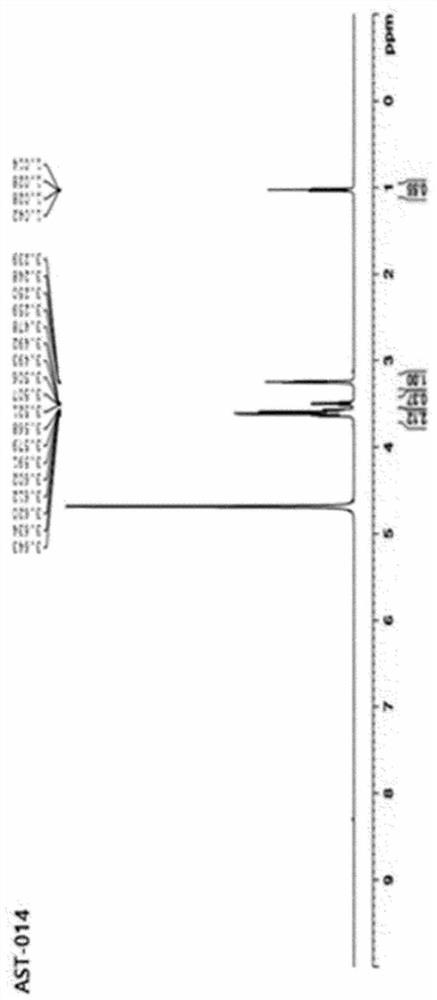
[0153] 1. Preparation of magnesium-serinate (Mg-Serinate)
[0154] 1.1. Preparation of magnesium-serinate (Mg-Serinate, AST-011)
[0155] Put 100 ml of distilled water in a 500 ml Erlenmeyer flask and heat up to a temperature of 70 to 80°C, then weigh 50 g (~0.48 moles) of L-serine (MW 105.1) in the distilled water while using a magnetic stirrer (magnetic stirrer) Stir to dissolve. After crushing MgO (MW 40.3) into small particles in a mortar, weigh 9.7g (~0.24moles) and add it in a small amount to L-serine aqueous solution while stirring at a temperature of 70~80°C. A reflux cooling device was attached in the medium, and the reaction was carried out for 2 hours under the same conditions.
[0156]In an uncooled state, centrifuge directly at 6000 rpm for 10 minutes, and recover 130 ml of supernatant. Ethanol was added to the supernatant so that the final concentration became 75 v / v%, and then stirred at room temperature for 14 hours with a magnetic stirrer to allow precipita...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com