Glipizide solid dispersion and preparation method thereof, glipizide solid dispersion tablet containing glipizide solid dispersion and preparation method thereof
A technology of solid dispersion and glipizide, which is applied in the direction of medical preparations of non-active ingredients, pharmaceutical formulas, active ingredients of sulfonylurea, etc., can solve the difficulties of large-scale production, poor stability of solid dispersions, grinding methods, etc. Low efficiency and other issues, to achieve the effects of no time-consuming cost, increased solubility and stability, and high Gibbs free energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation method of Example 1 includes the following steps:
[0068] (1) Weigh the raw material according to the formulation of the gyridazine solid dispersion described above, placing the Glielpyrazine, a unit carrier, and the binary vector to the beaker, resulting in a mixture, spare;
[0069] (2) Pour the solvent into the beaker of the mixture into the temperature of 20 ° C, the power is 40 kHz, and the power is stirred at a rate of 120 r / min; in the temperature of 55 ° C, the rotational speed is 80 rpm, rotation 40min The solid matter is obtained; wherein the solvent is mixed by a volume ratio of dichloromethane and methanol to obtain a mass of 1: 1 of the solvent is 6 times that of the total mass of the 1-membered vector and the binary vector.
[0070] (3) The solid material was placed in a vacuum drying tank at 25 ° C for 24 h, pulverized, 60 mesh sieve to obtain a gyropyrazine solid dispersion.
Embodiment 3
[0071] The preparation method of Example 3: Unlike Example 1, step (2) is: pouring the solvent into the beaker in the mixture at a temperature of 35 ° C, the power is 40 kHz, and the speed is 140 r / min. Ultrasound was stirred for 2 min; rotating at a temperature of 55 ° C, the rotation speed was 65 rpm, and the solid material was evaporated, to obtain a solid material; wherein the solvent was mixed by a volume ratio of dichloromethane and methanol. The volume of the solvent was Glops Zhenzine, one dollar carrier and the total mass of the binary vector.
[0072] Example 2, 4, 5, 6: Unlike Example 1, the step (2) is: pouring the solvent in a beaker in the mixture at a temperature of 25 ° C, the power is 40 kHz, and the speed is Ultrasound was stirred at 130R / min for 4 min; the temperature was stirred at a temperature of 50 ° C and a rotational speed of 70 rpm, and the solid material was obtained; wherein the solvent was mixed by a volume ratio of dichloromethane and methanol; so...
Embodiment 34
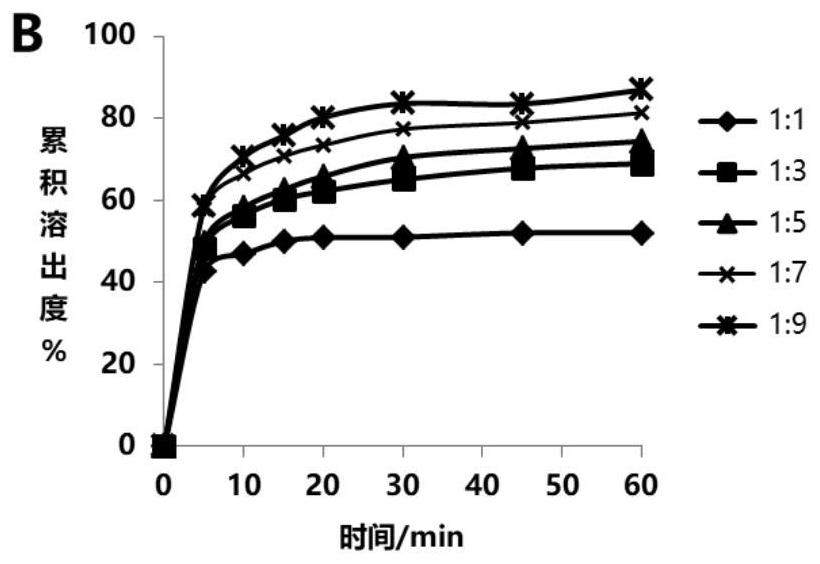
[0114] Example 34 Gullirazine solid dispersion sheet, Glielpyrazine (raw material pill) and the reference preparation Manduibao dissolution under pH 6.0. The results show that the Gullirazine solid dispersion has increased the dissolution of the raw material tablets; and the reference preparation Mandi Bao compared to F2 factor> 50, the dissolution has similarity (see Figure 9 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com