A semi-recombinant preparation method of glp-1 analogs
A technology of GLP-1 and analogs, which is applied in the field of DNA recombination and chemical preparation of fusion proteins, can solve the problems of cumbersome steps, low enzyme activity, and large usage, and achieve simple purification steps, high enzyme digestion efficiency, and enzyme use. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Construction of engineering strains of fusion protein 1
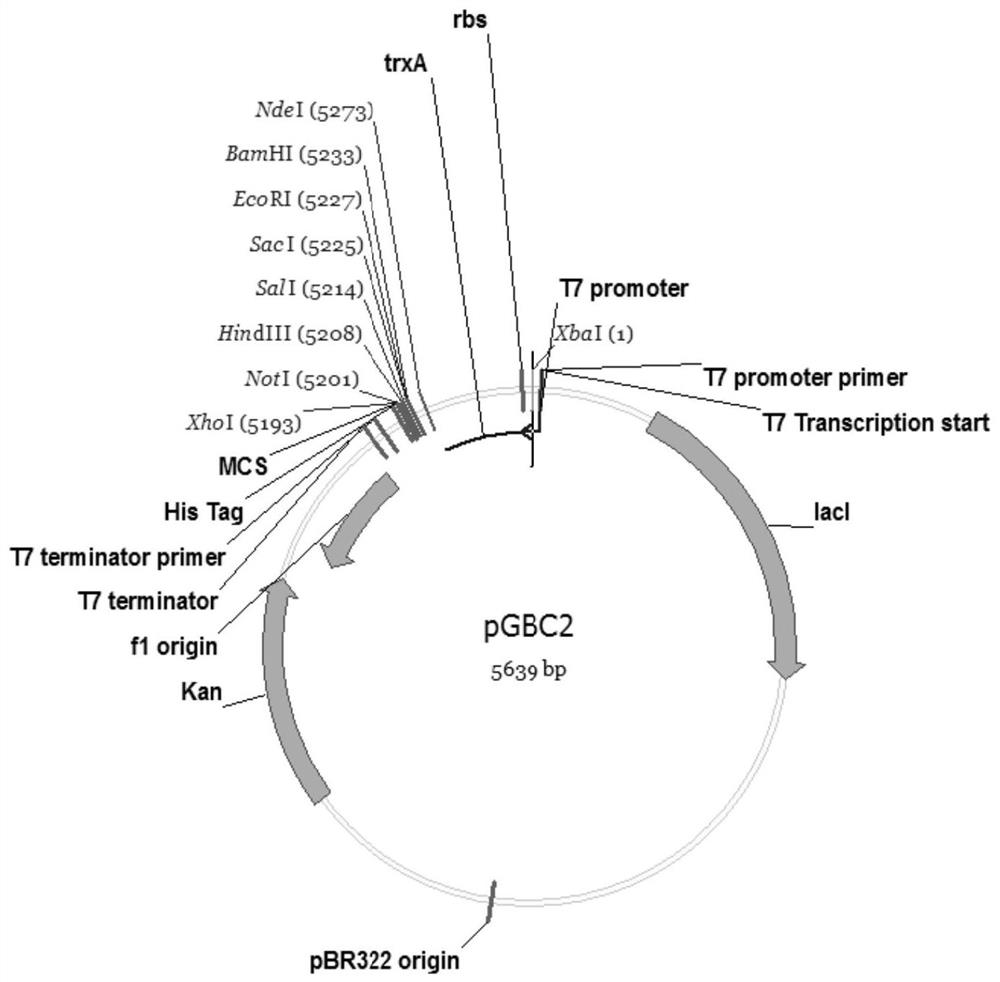
[0062] According to the amino acid sequence of fusion protein 1, according to codon degeneracy and codon preference of Escherichia coli, the coding gene sequence was designed, and the restriction enzyme NdeI restriction site CATATG was added at the 5' end of the gene, and a double stop was added at the 3' end The codon TAATGA and the restriction enzyme XhoI cut site CTCGAG to obtain the nucleotide sequence (as shown in SEQ ID NO: 1), and entrust the artificial synthesis of the nucleotide sequence, and then connect it to the plasmid pUC57 to obtain the recombinant plasmid pUC57-glp1 , saved in TOP10. Digest the plasmid with restriction enzymes NdeI and XhoI, recover the target gene, and connect it to the plasmid pGBC2 ( figure 1 ), transformed into Escherichia coli TOP10, and the correct recombinant plasmid pGBC2-glp1 was obtained by sequencing. Using the recombinant plasmid pGBC2-glp1 as a template, u...
Embodiment 2
[0068] Embodiment 2 constructs the engineering strain of fusion protein 2
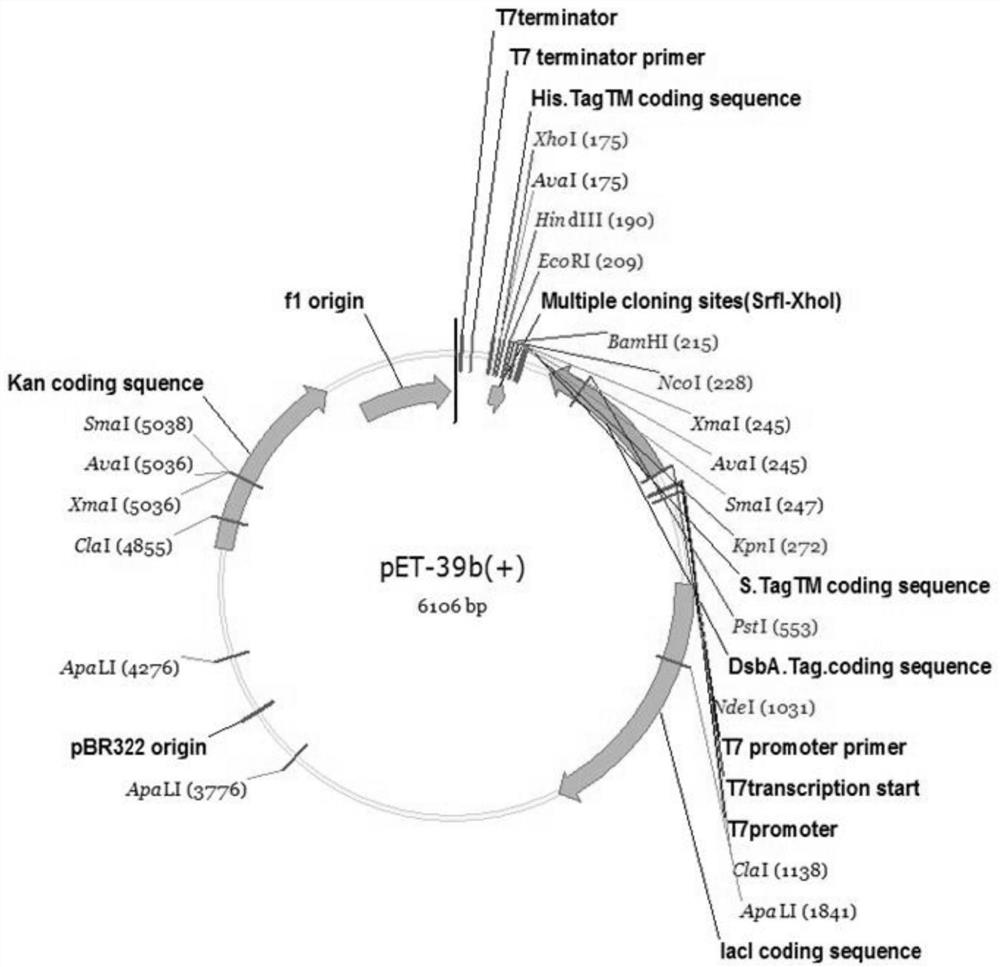
[0069] The nucleotide sequence of the gene encoding fusion protein 2 is shown in SEQ ID NO: 2. The construction method of the engineering strain BL21(DE3) / pET39b-glp2 capable of expressing fusion protein 2 is the same as that in Example 1.
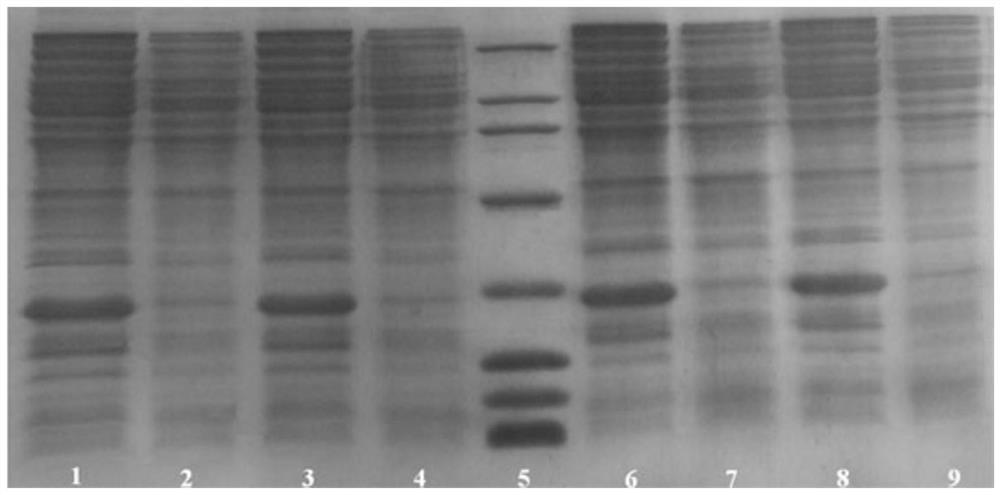
[0070] The SDS-PAGE electrophoresis of the expression product of engineering strain BL21(DE3) / pET39b-glp2 is shown in image 3 .
Embodiment 3
[0071] Embodiment 3 constructs the engineering strain of fusion protein 3
[0072] The nucleotide sequence of the gene encoding fusion protein 3 is shown in SEQ ID NO: 3, and the construction method of the engineering strain BL21(DE3) / pET39b-glp3 capable of expressing fusion protein 3 is the same as that in Example 1.
[0073] SDS-PAGE electrophoresis of the expression product of engineering strain BL21(DE3) / pET39b-glp3 see image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com