Phenylephrine hydrochloride and chlorphenamine maleate preparation and preparation method thereof
A technology of renin and preparations, which is applied in the field of renin pheniramine preparations and its preparation, can solve the problems of impurities generated by addition reactions, achieve high product stability, quickly control cold symptoms, and improve safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The embodiment of the present invention provides a preparation method of renin iramine preparation, which comprises the following steps:
[0048] S1. Provide the first core, the first coating material, the second core and the second coating material. The first core is phenylephrine or a pharmaceutically acceptable salt thereof, and the second core is malaysia Chlorpheniramine and / or brompheniramine maleate;
[0049] S2. Coating the first core with the first coating material to obtain the first coating;
[0050] S3. Coating the second core with the second coating material to obtain the second coating;
[0051] S4. Mixing the first coating and the second coating to obtain a reniniramine preparation.
[0052] In the preparation method provided in the examples of the present invention, the first coating is obtained by coating phenylephrine, and the second coating is obtained by coating chlorpheniramine maleate and / or brompheniramine maleate. Coating, so as to realize the...
Embodiment 1
[0061] This embodiment provides a kind of renin iramine preparation (capsule) and preparation method thereof, and the steps are as follows:
[0062] (11) Phenylephrine hydrochloride coating:
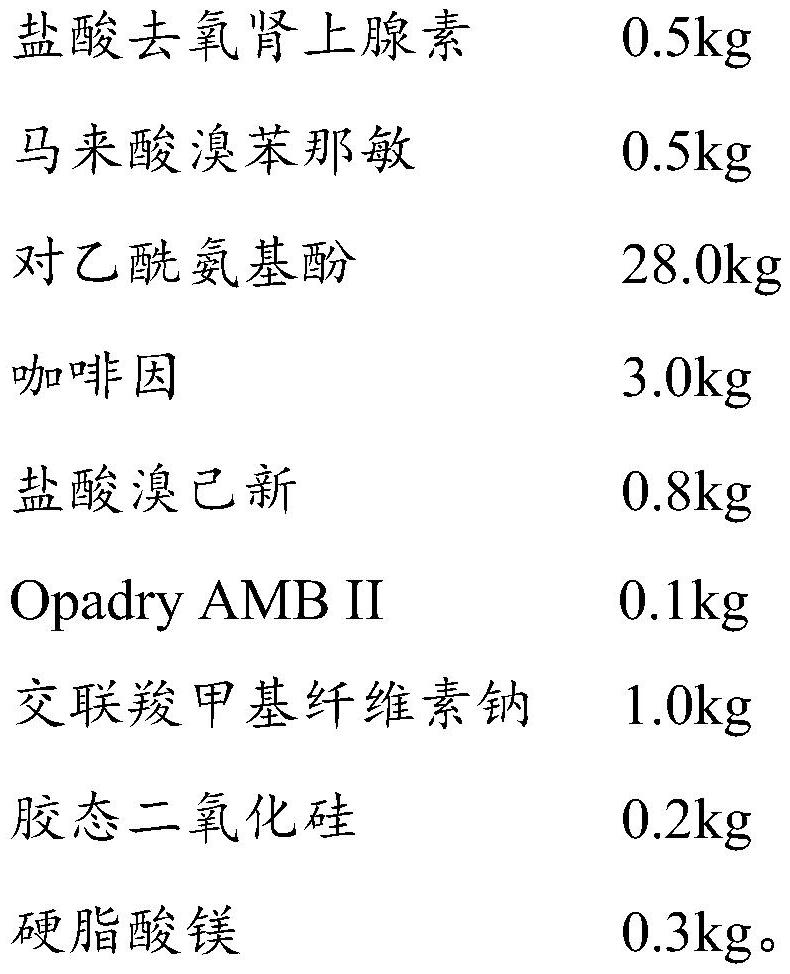
[0063] (111) coating liquid preparation: 0.05kg Opadry AMB II is dispersed in purified water, and the preparation solid content concentration is the coating liquid of 8%;
[0064] (112) Coating: add 0.5kg of phenylephrine hydrochloride (100-325 mesh) to the fluidized bed, open the coating, the air inlet temperature is 50°C, the atomization pressure is 1.0bar, and the air inlet volume is 20m 3 / h, under the condition that the spray speed is 5g / min, phenylephrine hydrochloride is coated. After the coating is completed, pass through a 60-mesh sieve for granulation to obtain phenylephrine hydrochloride coated granules;
[0065] (12) brompheniramine maleate coating:
[0066] (121) Coating solution preparation: 0.05kg Opadry AMB II is dispersed in purified water, and the coating solution who...
Embodiment 2
[0074] This embodiment provides a kind of renin iramine preparation (capsule) and preparation method thereof, and the steps are as follows:
[0075] (21) Phenylephrine hydrochloride coating:
[0076] (211) Coating solution preparation: disperse 0.15kg Opadry II in purified water, and prepare a coating solution with a solid content concentration of 10%;
[0077] (212) Coating: Add 0.5kg of phenylephrine hydrochloride (100-325 mesh) to the fluidized bed, open the coating, the air inlet temperature is 60°C, the atomization pressure is 3.0bar, and the air inlet volume is 100m 3 / h, under the condition that the spray speed is 30g / min, phenylephrine hydrochloride is coated. After the coating is completed, pass through a 60-mesh sieve for granulation to obtain phenylephrine hydrochloride coated granules;
[0078] (22) Chlorpheniramine maleate coating:
[0079] (221) Coating solution preparation: disperse 0.15kg Opadry II in purified water, and prepare a coating solution with a sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com