Preparation method of E-olopatadine
A technology of olopatadine and mass ratio, which is applied in the field of preparation of E-olopatadine, can solve the problems such as complex preparation process of E-olopatadine, and achieve the effect of reducing loss and reducing reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
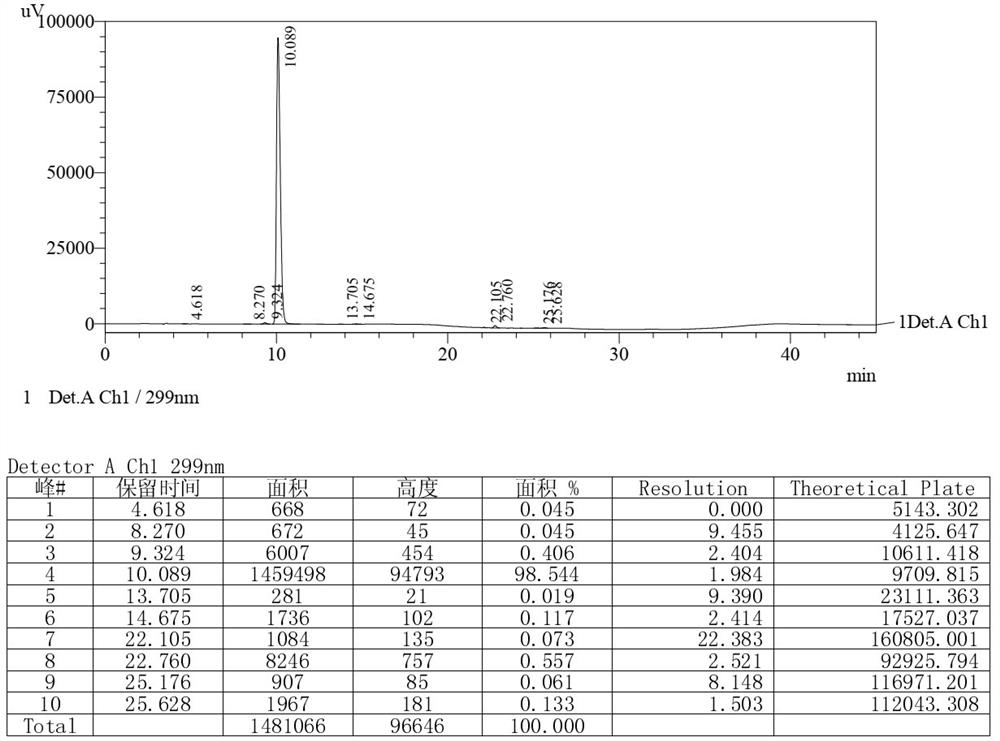
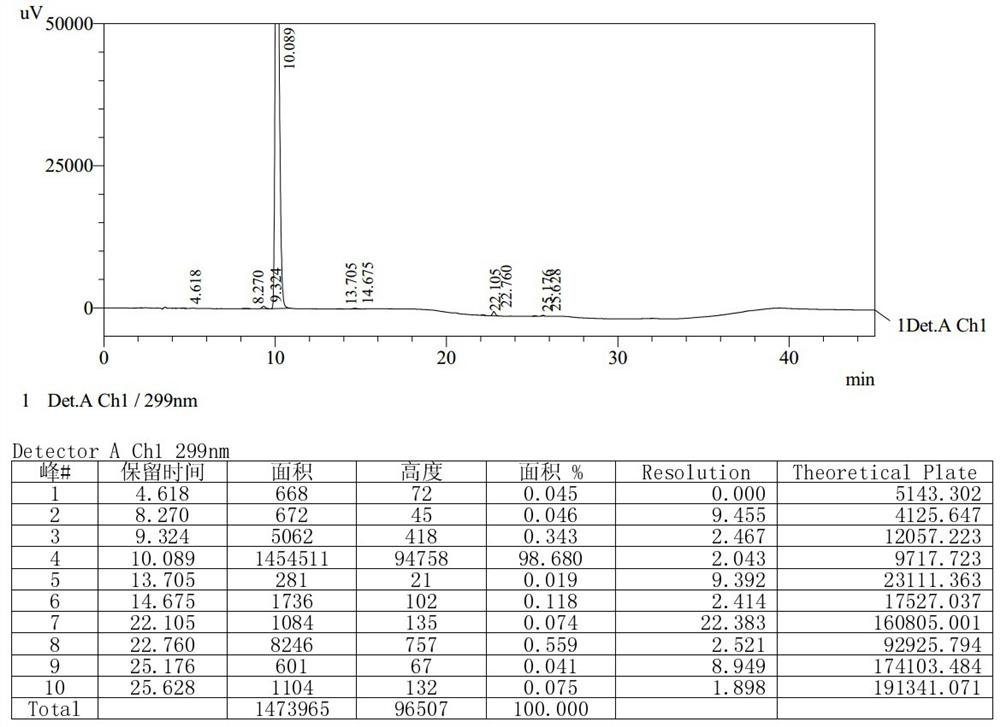
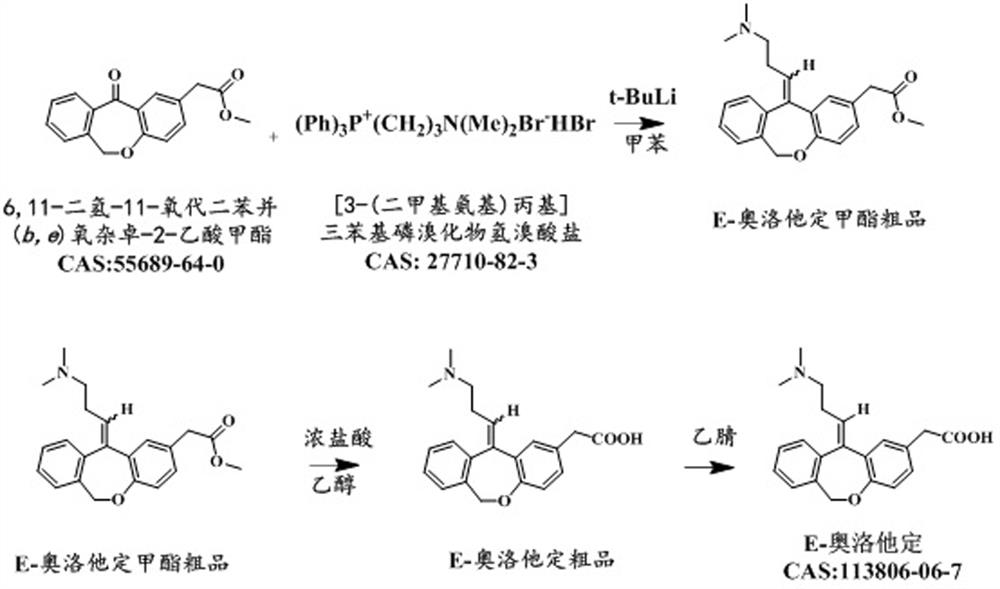
[0061] Under nitrogen protection, add 100g [3-(dimethylamino)propyl]triphenylphosphine bromide hydrobromide to the three-necked flask, then add 500g toluene, start stirring, control the temperature at about -8°C, and add it with a syringe Add 75ml of 1.3mol / L tert-butyllithium n-pentane solution, keep it warm for 1h after adding, and then slowly raise the temperature to 105~110℃. 50g of 6,11-dihydro-11-oxodibenzo( b,e ) The solution prepared by methyl oxazapine-2-acetate and 100g toluene was heated up to 105~110°C after dropping, stirred at 105~110°C for 3 hours, and then cooled to about 5°C. Control the temperature at about 5°C, slowly drop in 10g of methanol, then drop in 200g of 60% tetrahydrofuran aqueous solution, and finally add 800g of water. The aqueous layer of the reaction solution was adjusted to pH=6±0.2 with concentrated hydrochloric acid, then distilled to dryness under reduced pressure and evaporated to dryness to obtain a solid. The solid was passed through t...
Embodiment 2
[0065] Under nitrogen protection, add 120g [3-(dimethylamino)propyl]triphenylphosphine bromide hydrobromide to the three-necked flask, then add 500g toluene, start stirring, control the temperature at about -8°C, and add it with a syringe Add 83ml of 1.3mol / L tert-butyllithium n-pentane solution, keep it warm for 1.5h after adding, and then slowly raise the temperature to 105~110℃. 50g of 6,11-dihydro-11-oxodibenzo( b,e ) Oxazepine-2-acetic acid methyl ester and 100g of toluene, the temperature was raised to 105~110°C after dropping, stirred at 105~110°C for 3.5 hours, and then cooled to about 8°C. Control the temperature at about 8°C, slowly drop in 10g of methanol, then drop in 240g of 50% tetrahydrofuran aqueous solution, and finally add 750g of water. The aqueous layer of the reaction solution was adjusted to pH=6±0.2 with concentrated hydrochloric acid, then distilled to dryness under reduced pressure and evaporated to dryness to obtain a solid. The solid was passed thr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com