Application of beta 3-adrenergic receptor agonist in preparation of medicine for treating brain injury
A technology of epinephrine and therapeutic drugs, applied in the field of medicine, can solve the problem of lack of effective surgical or recognized drug treatment methods for cerebral hemorrhage, and achieve the effect of inhibiting focal brain inflammation and edema
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
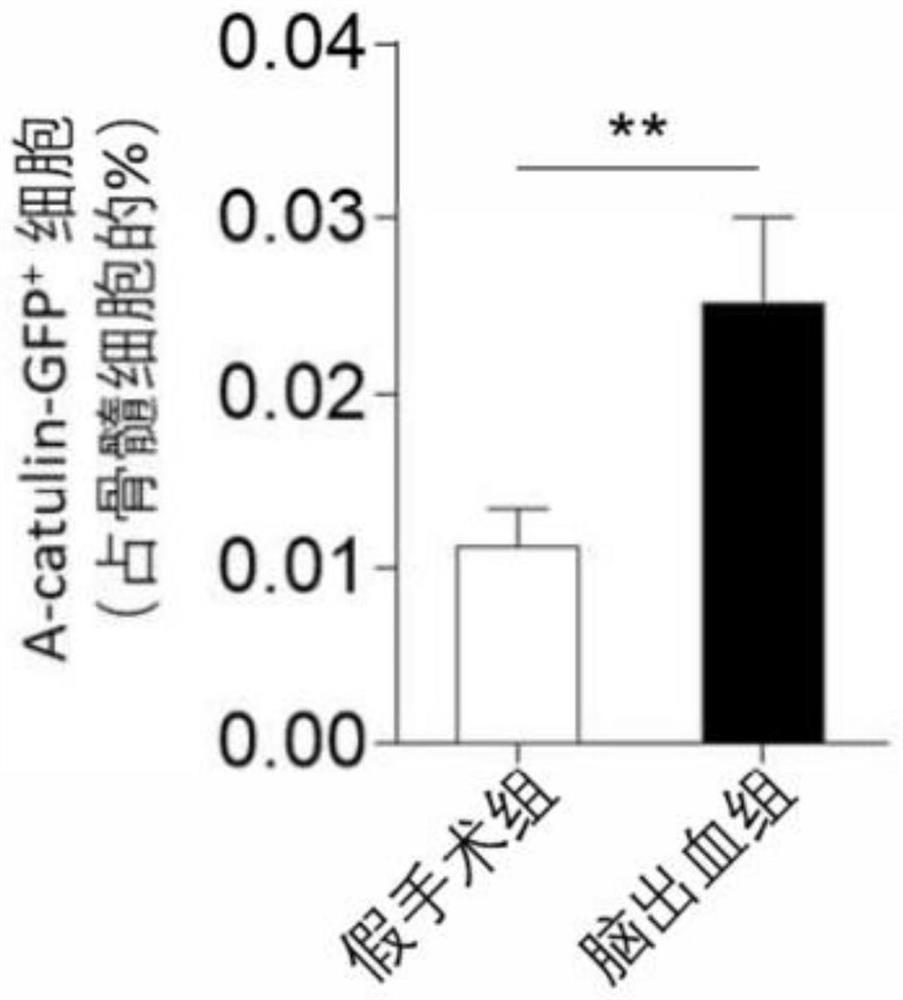
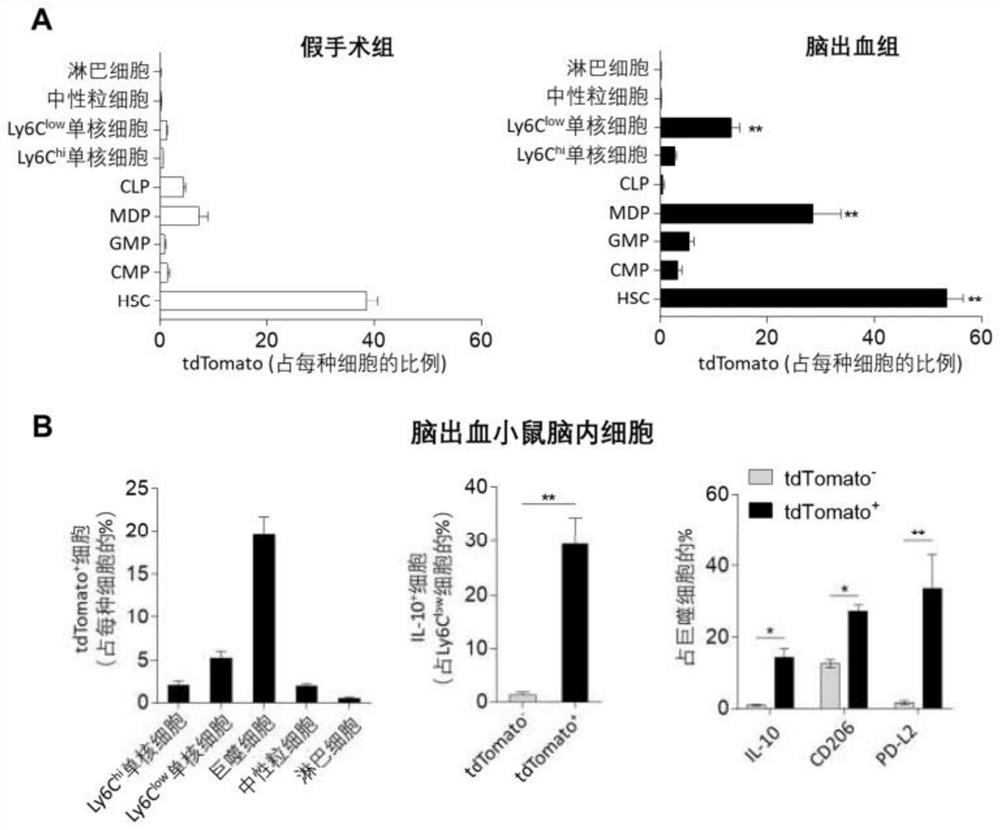
[0071] Embodiment 1: detect the cell (Ly6C of cerebral hemorrhage model mouse low , HSCs) and protein (Cdc42) changes
[0072] 1. Experimental animals:
[0073] B6. Cg-Gt(ROSA)26S, Fgd5-CreERT2, α-catulin GFP , Adrb3- / - mice, 3-4 months old, male.
[0074] 2. Model establishment:
[0075] Fgd5-CreERT2 mice were crossed with B6.Cg-Gt(ROSA)26S mice to obtain Fgd5-CreER-tdTomato mice. All mutant mice were backcrossed to the B6 background for at least 12 generations.
[0076] After mice were anesthetized by intraperitoneal injection of ketamine / xylazine, the mouse head was placed in a stereotaxic apparatus. After disinfecting and exposing the skull, drill a hole on the right side of the skull. The hole diameter needs to be able to insert a 30g needle, and the coordinates are 2.3mm to the right and 0.5mm to the front. For the autologous blood model, non-heparinized blood was drawn from the mouse corneal vein after anesthesia. Blood was then injected using a microsyringe wi...
Embodiment 2
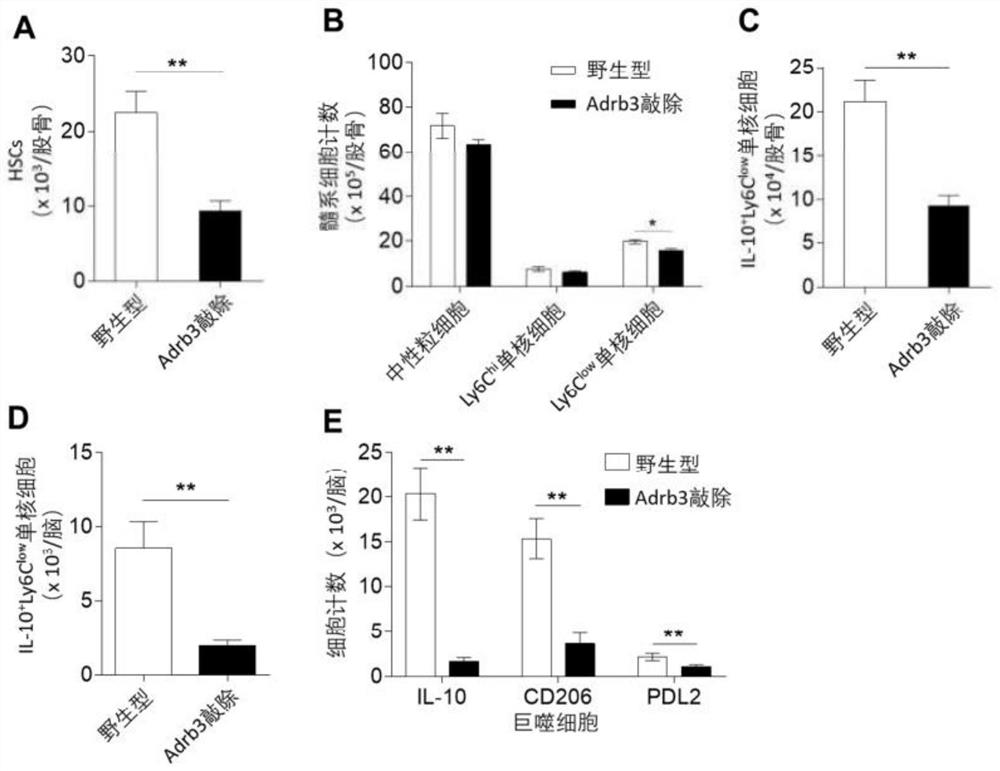
[0084] Example 2: Detection of changes in blood cells and brain damage in cerebral hemorrhage model mice after administration of mirabegron
[0085] 1. Experimental animals:
[0086] C57BL / 6 mice, 3-4 months old, male, were randomly divided into mirabegron administration group and control group.
[0087] 2. Model establishment:
[0088] After anesthetized by intraperitoneal injection of ketamine / xylazine, place the mouse head in a stereotaxic apparatus. After disinfecting and exposing the skull, drill a hole on the right side of the skull. The hole diameter needs to be able to insert a 30g needle, and the coordinates are 2.3mm to the right and 0.5mm to the front. For the autologous blood model, non-heparinized blood was drawn from the mouse corneal vein after anesthesia. Blood was then injected using a microsyringe with a 30 g needle. Insert the needle into the 3.7mm deep hole. After 15 minutes, 30 μL of autologous blood was injected into the brain parenchyma at a rate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com