Nanopore assemblies and uses thereof
A technology of nanopores and components, applied in the field of nanopores
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] This example describes the materials and methods used in the subsequent examples.
[0141] Materials and methods
[0142] Non-membrane protein channel remodeling
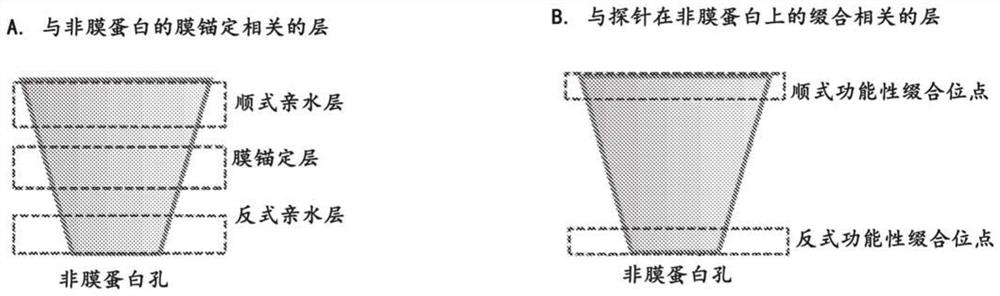
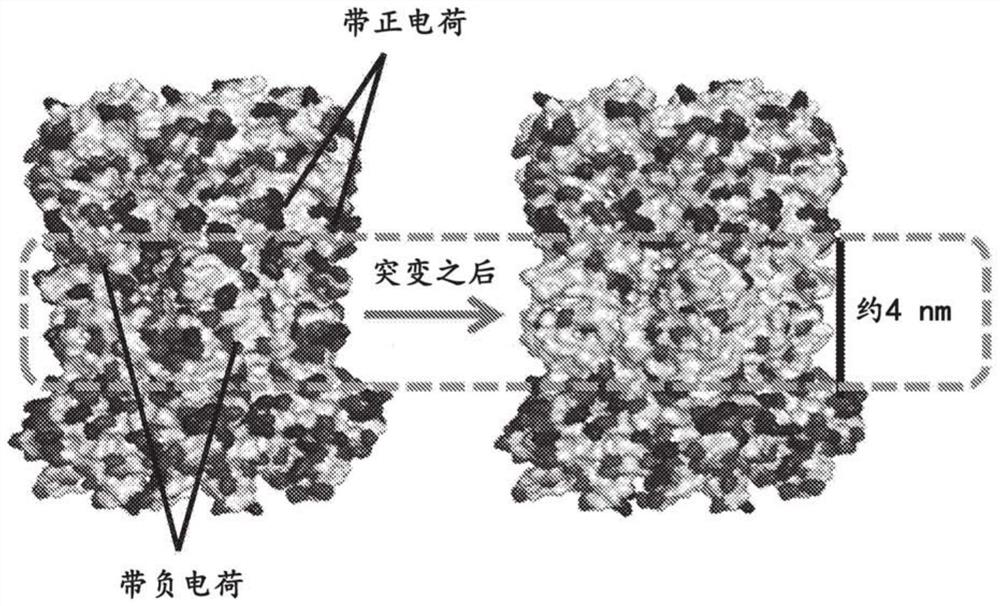
[0143]For non-membrane protein channels, direct insertion into lipid bilayers or polymer membranes is difficult because, unlike membrane protein channels, non-membrane protein channels typically lack a hydrophobic layer in the middle and hydrophilic layers at both ends, requiring extensive engineering . For use in biosensing or sequencing, non-membrane protein channels also need to be engineered and functionalized with different probes. Although nonmembrane protein channels may originate from a variety of sources with different sequences, shapes, structures, or properties, the strategies and methods used to reengineer them and enable their use as nanopores share some common features. As shown in Figures 1A and 1B, three distinct domains are important for membrane anchoring, and two regions are particularly...
Embodiment 2
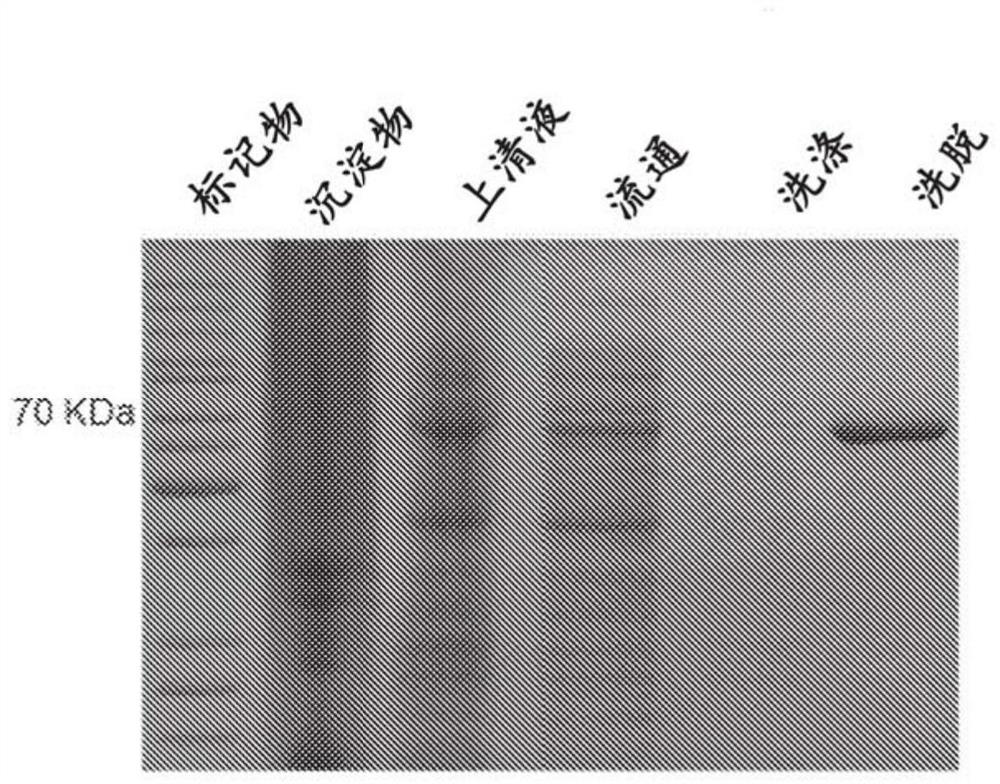
[0157] Expression and purification of phi29 gp-9 tail protein
[0158] Purified phi29 gp-9 tail protein and its variants in SDS PAGE in Figure 3 to Figure 4shown in . The expression and purification steps are as follows. The phi29 gp-9 tail protein gene and variant genes were cloned into the vector pBDHT with a His tag at the N-terminus. After transformation into BL21(DE3), one BL21 colony was inoculated in 5 mL of fresh LB medium with antibiotics inside and cultured at 37° C. in a shaker (220 rpm) for several hours until the OD reached 0.8. The flask was then kept at 4°C to cool. 0.5 mM IPTG was then added to the flask for induction. Bacteria were grown overnight at 16°C in a shaker (180 rpm). Bacteria were harvested for 10 min at 8000 rpm, the supernatant was discarded, and the pellet was then resuspended in lysis buffer (50 mM Tris pH 8.0, 500 mM NaCl). Sonicate the bacterial solution until the solution becomes clear and non-viscous. The solution was centrifuged at ...
Embodiment 3
[0160] Phage tail proteins as nanopores
[0161] Many phages contain long retractable or non-retractable tails or short non-retractable tails. The tail plays a key role in host cell recognition, membrane penetration, and viral genome ejection. Tail proteins are derived from, including but not limited to, phi29, T4, T3, T5, T7, SPP1, P22, P2, P3, lambda, mu, HK97, and Cl.
[0162] These protein channels of the invention are ideal for biosensing and sequencing of biomolecules such as disease-associated biomarkers, polynucleotide and polypeptide sequences. With improved membrane capacity, modified protein channels can be efficiently inserted into lipid or polymer membranes and act as nanopores for biosensing and sequencing. By conjugation with various probes, the modified protein channel has the ability to detect specific disease-associated biomarkers with high sensitivity and specificity. The pores of the invention may be present in homologous or heterologous pores.
[0163]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com