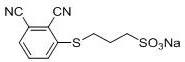
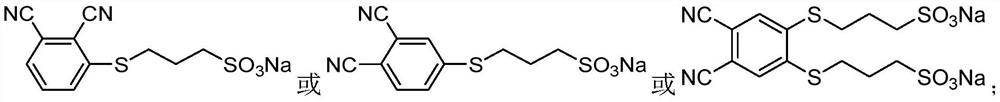
3-sulfopropane mercapto-modified phthalocyanine and its preparation method and application in pharmaceutical field
A sulfonic acid propane and sulfhydryl modification technology, which is applied in chemical instruments and methods, drug combinations, antitumor drugs, etc., can solve problems such as poor water solubility, and achieve the effects of stable properties, high antitumor activity, and easy storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
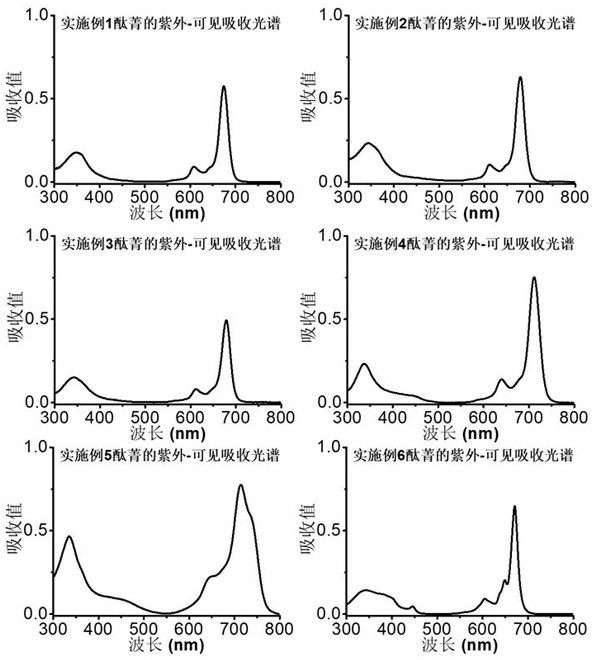
Image
Examples
Embodiment example 1
[0034] The synthesis of zinc phthalocyanine complexes substituted by β-position monosulfonic acid groups with the structure shown in the following formula:
[0035]
[0036] (1) prepare the phthalonitrile derivative of following formula of structure:
[0037] With 4-nitrophthalonitrile (10mmol) and sodium 3-mercaptopropanesulfonate (10-40mmol, preferably 20mmol) as reactants, dimethyl sulfoxide (DMSO) (20-100mL, preferably 30mL) as reactant Solvent, in the presence of potassium carbonate (30-90mmol, preferably 50mmol) and under the protection of nitrogen, stir the reaction at 20-45°C (preferably 45°C) for 17-24 hours, and monitor by thin layer chromatography. After the reaction, the reaction solution was suction filtered to remove unreacted potassium carbonate, the filtrate was poured into chloroform, part of the milky white precipitate was precipitated, filtered, washed with chloroform, acetone, and absolute ethanol respectively, the filter residue was collected, and vac...
Embodiment example 2
[0044] Synthesis of a disulfonic acid group substituted zinc phthalocyanine complex with the structure shown in the following formula
[0045]
[0046] (3) prepare the phthalonitrile derivative of following formula of structure:
[0047] With 4,5-dichlorophthalonitrile (10mmol) and sodium 3-mercaptopropanesulfonate (20-70mmol, preferably 30mmol) as reactants, DMSO (20-60mL) as solvent, potassium carbonate (30 In the presence of -90mmol, preferably 80mmol) and nitrogen protection, the reaction was stirred at 40-80°C (preferably 50°C) for 24-72 hours, and monitored by thin-layer chromatography. After the reaction, the reaction solution was suction filtered to remove unreacted potassium carbonate, the filtrate was poured into chloroform, part of the milky white precipitate was precipitated, filtered, washed with chloroform, acetone, and absolute ethanol respectively, the filter residue was collected, and vacuum-dried to obtain a white solid. The yield was 60%.
[0048] NM...
Embodiment example 3
[0054] Synthesis of α-position monosubstituted sulfonic acid zinc phthalocyanine complexes with the following structure:
[0055]
[0056] (5) Preparation of phthalonitrile derivatives with the following formula
[0057] With 3-nitrophthalonitrile (10mmol) and sodium 3-mercaptopropanesulfonate (10-40mmol, preferably 20mmol) as reactants, DMSO (20-100mL, preferably 30mL) as solvent, in potassium carbonate ( In the presence of 30-90mmol, preferably 50mmol) and nitrogen protection, the reaction was stirred at 20-45°C (preferably 45°C) for 17-24 hours, and monitored by thin-layer chromatography. After the reaction, the reaction solution was suction filtered to remove unreacted potassium carbonate, the filtrate was poured into chloroform, part of the milky white precipitate was precipitated, filtered, washed with chloroform, acetone, and absolute ethanol respectively, the filter residue was collected, and vacuum-dried to obtain a white solid. The yield was 75%.
[0058] NMR...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com