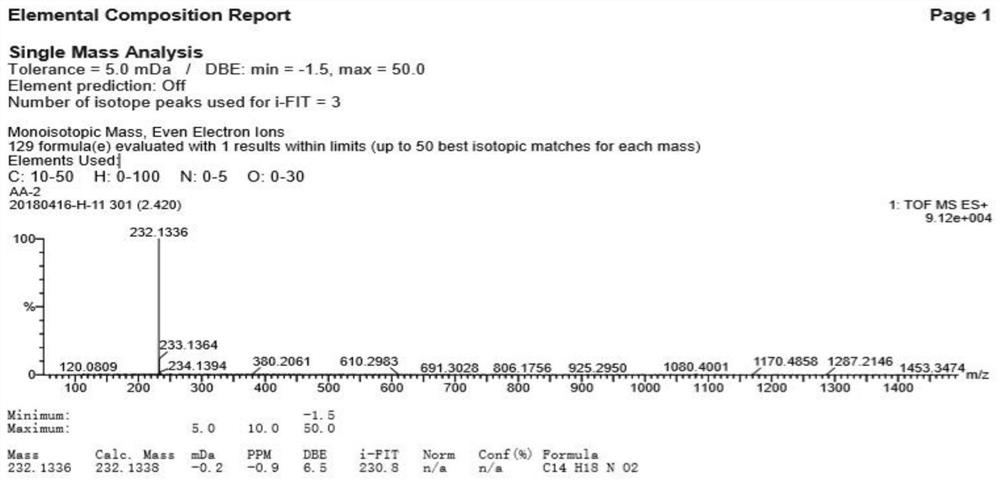
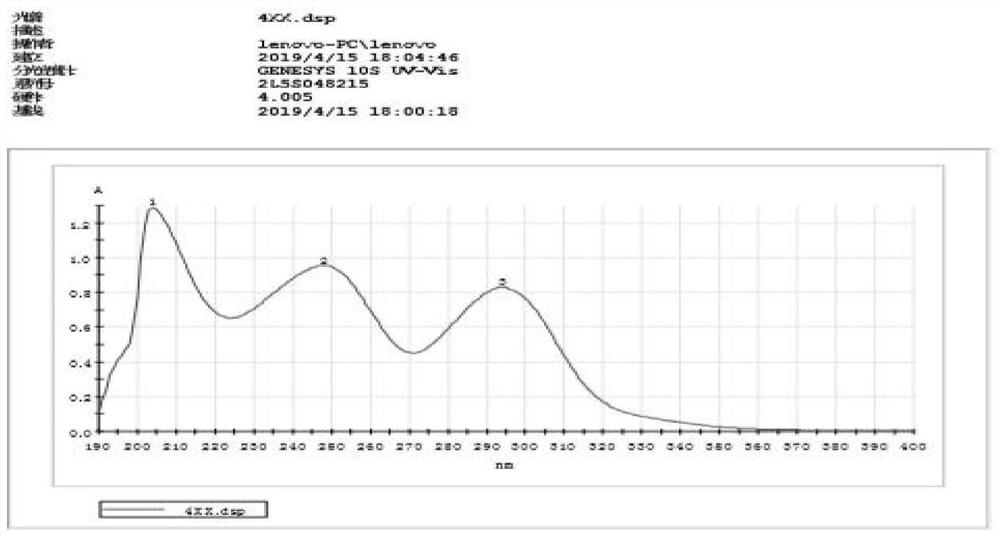
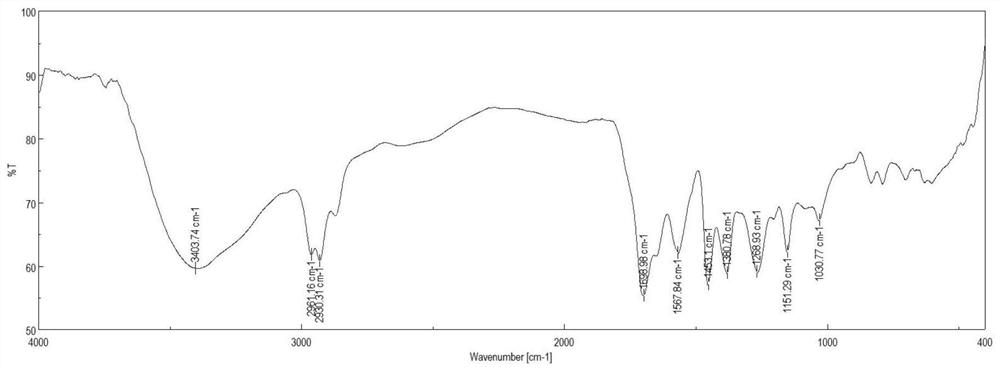
Alkaloid compound as well as preparation method and application thereof
A technology of alkaloids and compounds, applied in the field of medicine, can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 compound of the present invention
[0037] (1) Take the dry aerial part of Artemisia annua L. (the same below), extract twice with 3 times hydrothermal reflux, each time for 2 hours, combine the extracts, and recover the solvent under reduced pressure to 1 / 10 of the original volume , add 95% ethanol until the alcohol concentration reaches 80%, let it stand overnight, collect the supernatant and concentrate under reduced pressure to obtain the total extract. The total extract is dissolved in water, separated by HP-20 macroporous adsorption resin column chromatography, eluted with water, 45% ethanol, and 90% ethanol in sequence, and the eluents are collected respectively, concentrated under reduced pressure until there is no alcohol smell, and water Elution site, 45% ethanol elution site, 90% ethanol elution site;
[0038] (2) Take the 45% ethanol elution part of step (1), separate it by silica gel column chromatography, and use dichlorome...
Embodiment 2
[0040] The preparation of embodiment 2 compounds of the present invention
[0041] (1) Take the dry aerial part of Artemisia annua, extract twice with 4 times of hydrothermal reflux, each time for 2 hours, combine the extracts, recover the solvent under reduced pressure to 1 / 10 of the original volume, add 95% ethanol until the alcohol concentration reaches 80 %, let stand overnight, collect the supernatant and concentrate under reduced pressure to obtain the total extract. The total extract is dissolved in water, separated by HP-20 macroporous adsorption resin column chromatography, eluted with water, 50% ethanol, and 95% ethanol in sequence, and the eluents are collected respectively, concentrated under reduced pressure until there is no alcohol smell, and water Elution site, 50% ethanol elution site, 95% ethanol elution site;
[0042] (2) Separation by silica gel column chromatography, elution with dichloromethane-methanol gradient (100:0 to 0:100, v / v), 4 column volumes fo...
Embodiment 3
[0044] The preparation of embodiment 3 compounds of the present invention
[0045] (1) Take the dry aerial part of Artemisia annua, extract it with 4 times of hydrothermal reflux for 3 times, each time for 2 hours, combine the extracts, recover the solvent under reduced pressure to 1 / 10 of the original volume, add 95% ethanol until the alcohol concentration reaches 80 %, let stand overnight, collect the supernatant and concentrate under reduced pressure to obtain the total extract. The total extract is dissolved in water, separated by HP-20 macroporous adsorption resin column chromatography, eluted with water, 55% ethanol, and 100% ethanol in sequence, and the eluents are collected respectively, concentrated under reduced pressure until there is no alcohol smell, and water Elution site, 55% ethanol elution site, 100% ethanol elution site;
[0046] (2) Take the 55% ethanol elution part of step (1), separate it by silica gel column chromatography, and use dichloromethane-methan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com