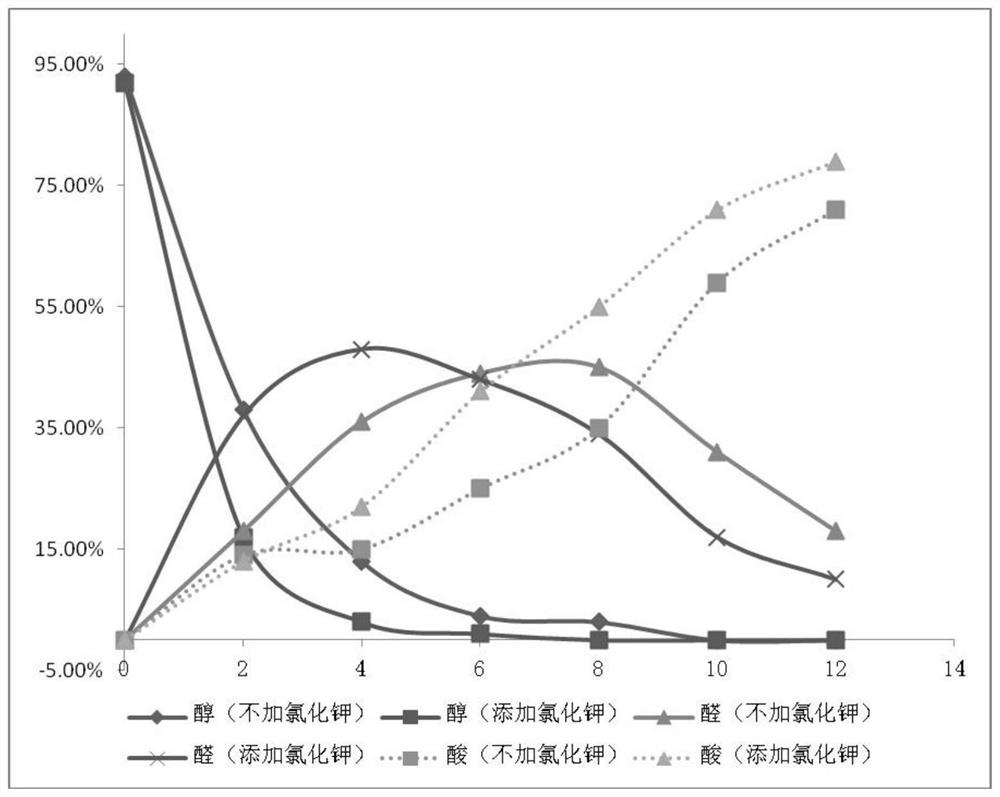
Method for synthesizing carboxylic acid or ketone compounds from alcohol or aldehyde by taking oxygen or oxygen in air as oxidant
A technology of ketone compounds and oxidants, which is applied in the direction of oxidative preparation of carbonyl compounds, preparation of organic compounds, organic chemical methods, etc., can solve the problem of low catalytic activity, and achieve the effects of environmental friendliness, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] Add ferric nitrate nonahydrate (401.8mg, 1.0mmol), 4-hydroxy-2,2,6,6-tetramethylpiperidine oxide (172.4mg, 1.0mmol), potassium chloride (75.9mg , 1.0mmol), 1a (1.4381g, 10.0mmol) and 1,2-dichloroethane (2mL), pumped gas (pure oxygen) three times, stirred at room temperature (25°C) for 33 hours, and obtained 49% 2a (with dibromomethane as internal standard, NMR yield), 39% of 3a (with dibromomethane as internal standard, NMR yield).
Embodiment 2
[0034]
[0035] Add ferric nitrate nonahydrate (405.8mg, 1.0mmol), 4-hydroxy-2,2,6,6-tetramethylpiperidine oxide (171.4mg, 1.0mmol), potassium chloride (75.7mg , 1.0mmol), 1a (1.4353g, 10.0mmol) and dichloromethane (2mL), exchanged gas (pure oxygen) three times, and stirred at room temperature (25°C) for 33 hours to obtain 41% of 2a (based on dibromomethane Internal standard, NMR yield), 27% of 3a (with dibromomethane as internal standard, NMR yield).
Embodiment 3
[0037]
[0038] Add ferric nitrate nonahydrate (403.5mg, 1.0mmol), 4-hydroxy-2,2,6,6-tetramethylpiperidine oxide (171.6mg, 1.0mmol), potassium chloride (75.0mg , 1.0mmol), 1a (1.4359g, 10.0mmol) and toluene (2mL), gas exchanged (pure oxygen) three times, and stirred at room temperature (25°C) for 24 hours to obtain 25% of 2a (with dibromomethane as internal standard , NMR yield), 63% of 3a (with dibromomethane as internal standard, NMR yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com