A kind of donkey milk-derived Pediococcus pentosacea and its application
A technology for Pediococcus pentosaceus and donkey milk, which is applied in the application, bacteria, dairy products and other directions, can solve the problems of preparing yogurt without disclosure of Pediococcus pentosaceus, achieves alleviation of lactose intolerance and bacterial diarrhea, improves the Immunity, improve the effect of intestinal microecological environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
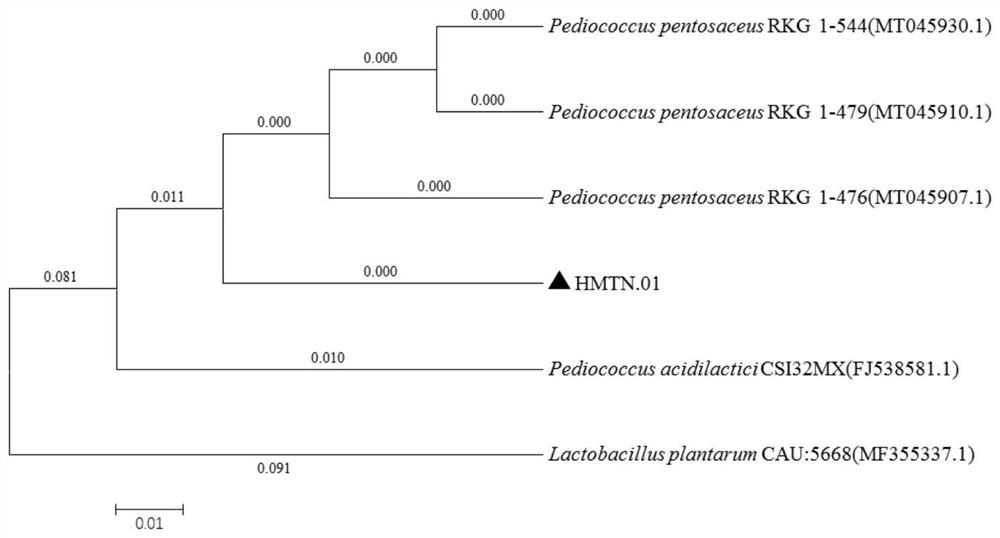
[0048] Screening and Identification of Example 1 Bacterial Strains
[0049] 1.1 Isolation and purification of strains
[0050] First, the improved MRS (containing 0.5g / L cysteine) medium was prepared and sterilized, and poured onto the plate until it was solidified, then the donkey milk sample collected from the Hami area of Xinjiang was diluted with sterile normal saline, and the Dilution gradient of 10 -2 、10 -3 、10 -4 、10 -5 、10 -6 100 μL of diluent was sucked and coated on the solidified plate, and the number of the plate was recorded, and the coated plate was placed in an anaerobic box at 37°C and incubated upside down for 36 hours.
[0051] For each donkey milk sample, select a flat plate with appropriate dilution to pick single colonies with different colors, sizes and shapes for microscopic examination, keep the colonies with rod-shaped or spherical cells, and continuously transfer and streak culture for 3 times, and then pick The pure single colony is placed o...
Embodiment 2
[0066] Example 2 Study on the probiotic properties of Pediococcus pentosaceae HMTN.01
[0067] 2.1 Acid tolerance and bile salt tolerance of Pediococcus pentosaceae HMTN.01
[0068] (1) Determination of acid tolerance of Pediococcus pentosaceae HMTN.01
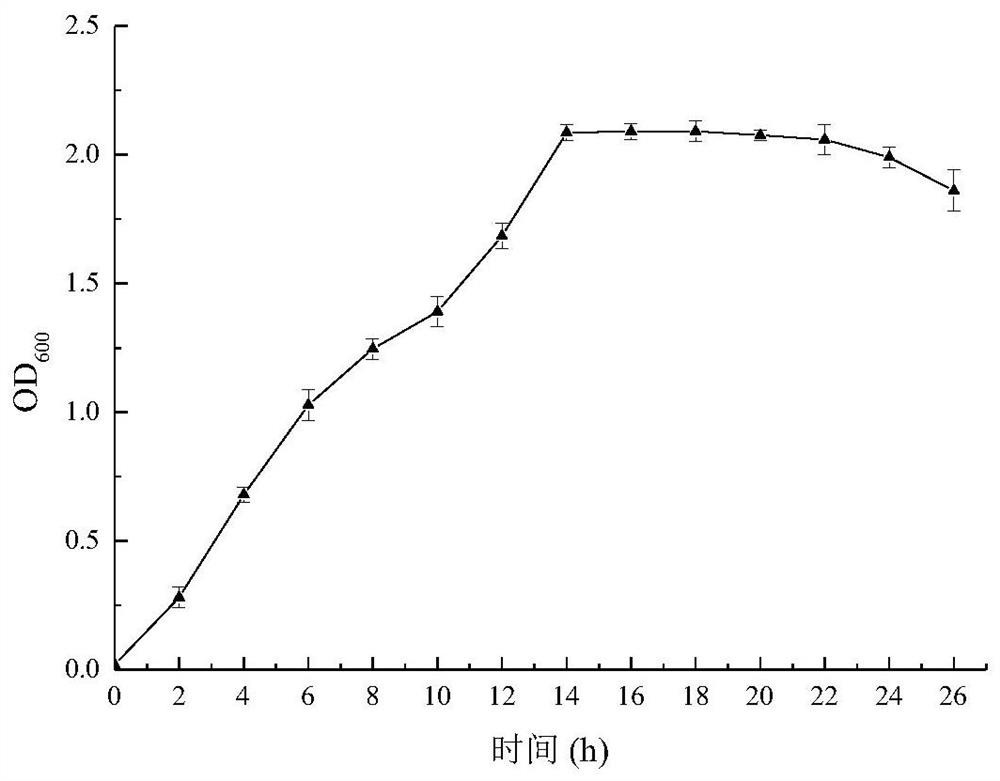
[0069] After activation, Pediococcus pentosaceae HMTN.01 was inoculated in liquid MRS medium with an inoculum of 1%, and after static culture at 37°C for 18 hours, the bacterial concentration was about 4.8×10 8 CFU / mL, after centrifugation at 4000r / min for 10min, the bacteria were collected, washed twice with sterile saline, and then resuspended in liquid MRS medium at pH 2.5. At 0, 1, 2, and 3 hours, draw 100 μL of the bacterial suspension and use sterile saline for gradient dilution, and select an appropriate dilution to count the colonies on the plate. The strain in the control group was Streptococcus thermophilus D-2, and the acid tolerance experiment was the same as above, and all were parallelized 3 times.
[0070] (2...
Embodiment 3
[0108] The determination of embodiment 3 bacterial strain drug resistance
[0109] Both the resistance and infection ability of probiotics to antibiotics have a great impact on human safety. When probiotics antagonize pathogenic bacteria, if the drug resistance gene is transferred to the pathogenic bacteria, the pathogenic bacteria will develop resistance to the corresponding antibiotics. While lactic acid bacteria are widely recognized as safe, their safety needs to be evaluated.
[0110] According to the drug susceptibility test method recommended by WHO, the drug susceptibility test of 20 kinds of antibiotics was carried out on Pediococcus pentosaceae HMTN.01 by Kirby-Bauer method (K-B disc diffusion method). Referring to NCCLS regulations, dilute the inoculum to 10 7 ~10 8 CFU / mL, take 120 μL of the bacterial suspension on the surface of the MRS agar plate, spread it evenly, and stick the tablet on the surface with sterile tweezers after the surface of the plate is dry....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com