Cryoprotectant capable of being directly transfused back for stem cells and related products thereof
A technology for stem cells and cryopreservation solution, applied in the field of cryopreservation agents, can solve the problems of self-renewal of cultured stem cells and uncertain factors in the differentiation process, avoid uncertain factors and contamination of cultured cells, avoid inducing immune responses, The effect of increasing production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Cryopreservation, recovery and characteristic detection of umbilical cord mesenchymal stem cells
[0040] 1. Experimental materials
[0041] Umbilical cord mesenchymal stem cells, DMSO, compound electrolyte injection, 5% glucose injection, 20% human serum albumin injection, low molecular weight dextran injection, Thermo CO 2 Incubator, Thermo centrifuge, biological safety cabinet, Accuri C6plus flow cytometer, liquid nitrogen tank.
[0042] 2. Test method
[0043] Example 1
[0044] Preparation of cryopreservation solution: in parts by volume, measure 6 parts of DMSO, 32 parts of compound electrolyte injection, 32 parts of 5% glucose injection by mass fraction, 18 parts of 20% human serum albumin injection by mass fraction, 12 parts of low-molecular-weight dextran injection, the components are mixed and shaken evenly.
[0045] The compound electrolyte injection contains 5.26g of sodium chloride, 5.02g of sodium gluconate, 3.68g of sodium acetate, 0.37g of ...
Embodiment 2
[0056] Preparation of cryopreservation solution: in parts by volume, measure 5 parts of DMSO, 30 parts of compound electrolyte injection, 30 parts of 5% glucose injection by mass fraction, 25 parts of 20% human serum albumin injection by mass fraction, 10 parts of low-molecular-weight dextran injection, the components are mixed and shaken evenly.
[0057] The compound electrolyte injection contains 5.26g of sodium chloride, 5.02g of sodium gluconate, 3.68g of sodium acetate, 0.37g of potassium chloride and 0.30g of magnesium chloride per 1000mL.
Embodiment 3
[0059] Preparation of cryopreservation solution: in parts by volume, measure 10 parts of DMSO, 35 parts of compound electrolyte injection, 35 parts of 5% glucose injection by mass fraction, 10 parts of 20% human serum albumin injection by mass fraction, 10 parts of low-molecular-weight dextran injection, the components are mixed and shaken evenly.
[0060] Other operation steps of embodiment 2 and embodiment 3 are identical with embodiment 1, also can obtain conclusion of the present invention.
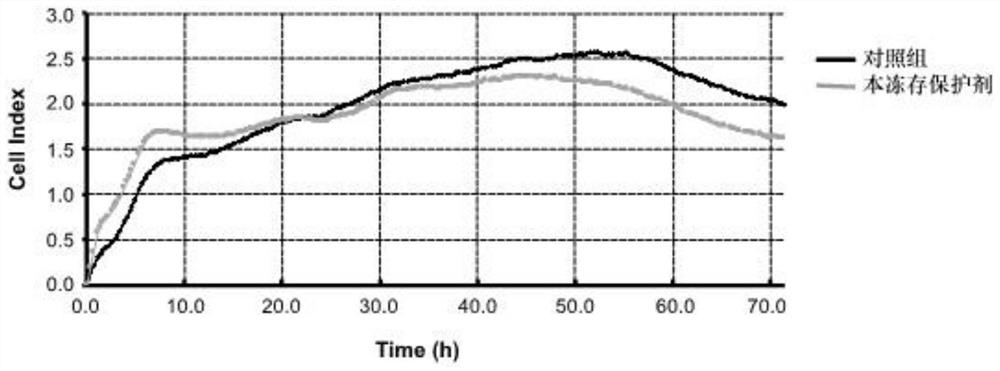
[0061] Using the accelerated destruction experiment to measure the shelf life of the cryoprotectant of the present invention, the shelf life of the cryoprotectant of the present invention is measured to be 2 years, and the cryoprotectant of the present invention can be used to directly cryopreserve mesenchymal stem cells within two years , and the mesenchymal stem cells can be recovered after being cryopreserved for a certain period, and can be directly injected and used, that is, dir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com