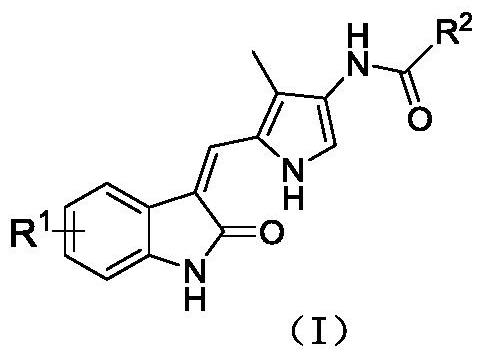
4-methylpyrrole substituted indolone derivative and preparation method and medical application thereof
A technology of methylpyrrole and indolinone, applied in the field of indolinone derivatives or their pharmaceutically acceptable salts, can solve problems such as gaps, low chemical stability, and decreased purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] (S,Z)-N-(5-((5-fluoro-2-oxindol-3-ylidene)methyl)-4-methyl-1H-pyrrol-3-yl)pyrrolidin-2- Synthesis of Formamide Hydrochloride (A01)
[0072]
[0073] Synthesis of intermediate III: at 0°C, POCl 3 (1 mL) was slowly added dropwise to anhydrous DMF (1.1 mL), and after stirring at room temperature for 0.5 hours, a DMF (2 mL) solution of compound II (0.80 g, 6.35 mmol) was added to the reaction solution, and then heated to 80°C for 0.5 hours. After the LC-MS monitoring reaction is complete, stop heating, cool to room temperature, under ice bath cooling, use saturated Na 2 CO 3 Solution Adjust the pH value of the reaction solution to 7, then extract with dichloromethane (20 mL×2), combine several layers, wash with water and saturated sodium chloride solution successively, and dry over anhydrous sodium sulfate. Suction filtration, concentration of the filtrate, and purification by column chromatography (petroleum ether: ethyl acetate = 20:1-4:1) gave 0.79 g of a white sol...
Embodiment 2
[0083] (R,Z)-N-(5-((5-fluoro-2-oxindol-3-ylidene)methyl)-4-methyl-1H-pyrrol-3-yl)pyrrolidin-2- Synthesis of Formamide Hydrochloride (A02)
[0084]
[0085] Synthesis of intermediate VII-2: Compound VI-1 (0.40g, 1.55mmol) and Boc-D-proline (0.37g, 1.71 mmol) were dissolved in DMF (50mL), and the condensing agent PyBOP (1.05g, 2.02mmol) and DIPEA (0.77mL, 4.66mmol), react at room temperature for 12 hours. After the completion of the reaction as monitored by TLC, the reaction solution was added to water (50 mL), extracted with ethyl acetate (30 mL×2), the organic layers were combined, washed once with water and sodium chloride solution, and dried over anhydrous sodium sulfate. Suction filtration, concentration of the filtrate, and purification by column chromatography (dichloromethane:methanol=50:1~20:1) gave 0.53 g of a yellow solid with a yield of 75%, which was directly used in the next reaction.
[0086]
[0087] Synthesis of Compound A02: Compound VII-2 (100 mg, 0.22...
Embodiment 3
[0089] (R,Z)-N-(5-((5-fluoro-2-oxindol-3-ylidene)methyl)-4-methyl-1H-pyrrol-3-yl)pyrrolidin-3- Synthesis of Formamide Hydrochloride (A03)
[0090]
[0091] Synthesis of Intermediate VII-3: Dissolve compound VI-1 (0.40 g, 1.55 mmol) and (R)-1-Boc-3-carboxypyrrolidine (0.37 g, 1.71 mmol) in DMF (50 mL), add A mixture of PyBOP (1.05g, 2.02mmol) and DIPEA (0.77mL, 4.66mmol) was reacted at room temperature for 12 hours. After the completion of the reaction as monitored by TLC, the reaction solution was added to water (50 mL), extracted with ethyl acetate (30 mL×2), and the organic layers were combined, washed with water and sodium chloride solution successively, and dried over anhydrous sodium sulfate. After suction filtration, the filtrate was concentrated, and purified by column chromatography (dichloromethane:methanol=50:1~20:1) to obtain 0.54 g of a yellow solid with a yield of 77%, which was directly used in the next reaction.
[0092]
[0093] Synthesis of Compound A0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com