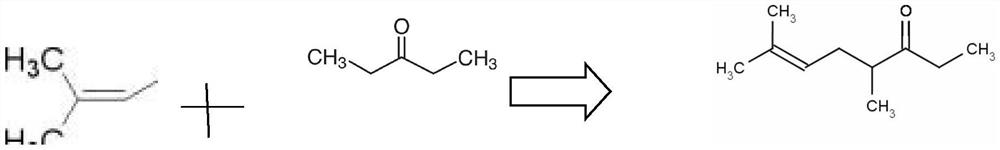
Synthesis method of dimethyl octenone
A technology for the finished product of dimethyl octenone and dimethyl octenone, which is applied to the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, etc., can solve problems such as poor safety and no reported synthesis, and achieve Effects of mild conditions, less pollution, and simple process routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 25.8g of 3-pentanone, 64g of NaOH solution with a mass fraction of 50%, and 0.6g of trioctylmethylammonium chloride into a stirred four-necked flask, stir and heat up to 30°C, and dropwise add 3,3-di 14.9g of methallyl bromide, the dropping rate is 12ml / h, after the dropwise addition, continue to stir for 1h, take a sample for detection, all 3,3-dimethylallyl bromide has reacted, stop stirring, let stand and separate , to obtain an aqueous phase and an organic phase, the aqueous phase was extracted 3 times with 25.8g 3-pentanone to obtain an organic phase, and then the organic phase was combined with the organic phase obtained by the reaction; the 3-pentanone solvent was first distilled out, and then the vacuum distillation Dimethyl octenone was extracted to obtain 12.6 g of finished product (95%) of dimethyl octenone, and the yield of dimethyl octenone was 82%.
Embodiment 2
[0033] Add 25.8g of 3-pentanone, 64g of KOH solution with a mass fraction of 50%, and 0.6g of trioctylmethylammonium chloride into a stirred four-neck flask, stir and raise the temperature to 25°C, and dropwise add 3,3-di 14.9g of methallyl bromide, the dropping rate is 12ml / h, after the dropwise addition, continue to stir for 1h, take a sample for detection, all 3,3-dimethylallyl bromide has reacted, stop stirring, let stand and separate , to obtain an aqueous phase and an organic phase, the aqueous phase was extracted 3 times with 25.8g 3-pentanone to obtain an organic phase, and then the organic phase was combined with the organic phase obtained by the reaction; the 3-pentanone solvent was first distilled out, and then the vacuum distillation Dimethyl octenone was extracted to obtain 12.8 g of finished product (95%) of dimethyl octenone, and the yield of dimethyl octenone was 83.1%.
Embodiment 3
[0035] Add 25.8g of 3-pentanone, 64g of NaOH solution with a mass fraction of 50%, and 0.8g of benzyltriethylammonium chloride into a stirred four-necked flask, stir and heat up to 30°C, and dropwise add 3,3-diethylammonium chloride 14.9g of methallyl bromide, the dropping rate is 12ml / h, after the dropwise addition, continue to stir for 1h, take a sample for detection, all 3,3-dimethylallyl bromide has reacted, stop stirring, let stand and separate , to obtain an aqueous phase and an organic phase, the aqueous phase was extracted 3 times with 25.8g 3-pentanone to obtain an organic phase, and then the organic phase was combined with the organic phase obtained by the reaction; the 3-pentanone solvent was first distilled out, and then the vacuum distillation Dimethyl octenone was extracted to obtain 12.1 g of finished product (95%) of dimethyl octenone, and the yield of dimethyl octenone was 78.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com