Lidocaine hydrochloride gel and preparation method thereof
A technology of lidocaine hydrochloride and gel, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to achieve large drug loading, avoid vascular bleeding, and avoid irreversible damage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
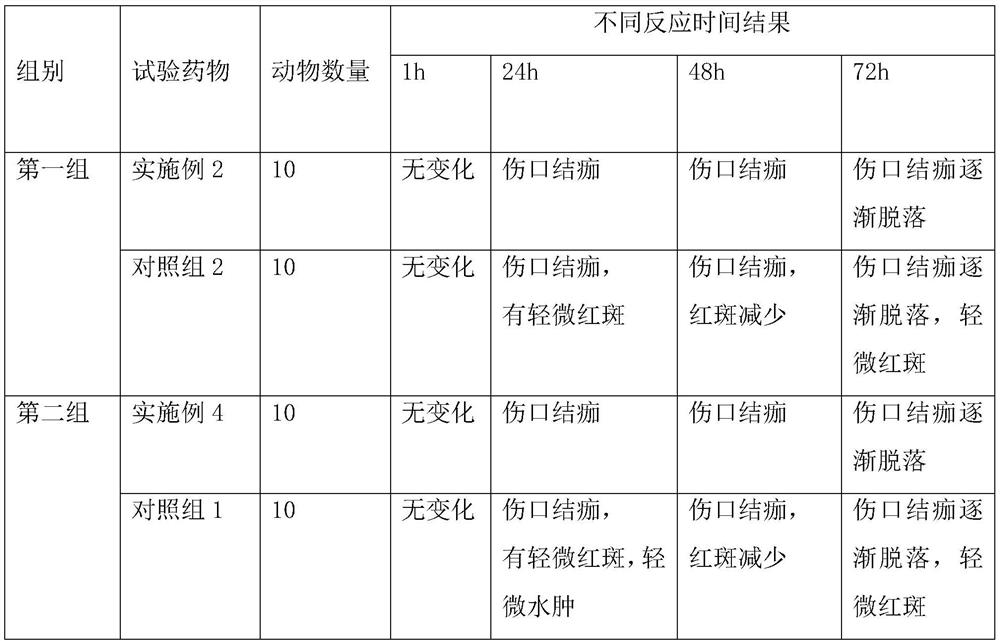
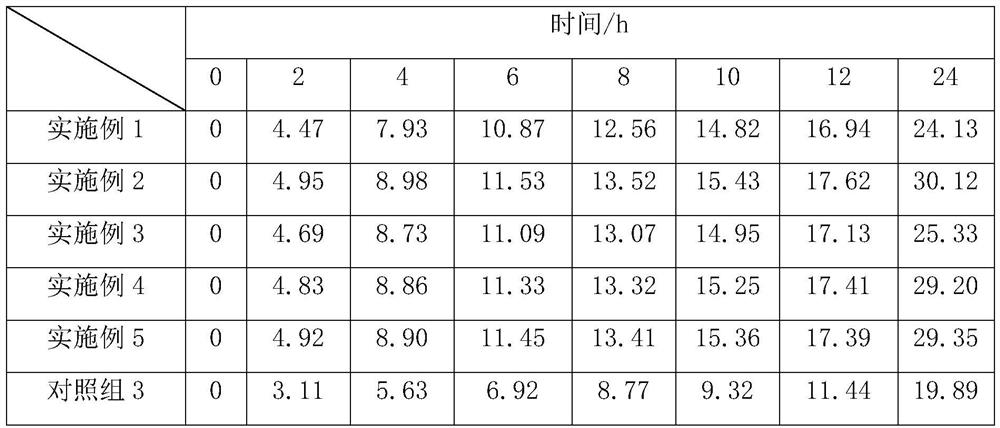
[0042] This embodiment relates to a lidocaine hydrochloride gel and a preparation method thereof, which consists of the following components in parts by weight: 0.2 parts of lidocaine hydrochloride, 0.2 parts of xanthan gum as a gel matrix, hypromellose 0.2 part of E4M, 0.01 part of 0.1M sodium hydroxide, 8 parts of water for injection, 0.1 part of vitamin P, 0.2 part of eucalyptus oil, 0.5 part of Span 65, 1 part of Tween 80, 0.4 part of polyethylene glycol 400 ;
[0043] A preparation method for lidocaine hydrochloride gel, comprising the following steps:
[0044] a, extract eucalyptus oil;
[0045] B, the eucalyptus oil that step a extracts is mixed with Span 65, Tween 80, polyethylene glycol 400;
[0046] c. Dissolve an appropriate amount of vitamin P in water for injection to obtain a vitamin P aqueous solution; then weigh an appropriate amount of gel matrix and add vitamin P aqueous solution to dissolve, and finally add 0.1M sodium hydroxide to adjust the pH value to 6...
Embodiment 2
[0050] This embodiment relates to a lidocaine hydrochloride gel and a preparation method thereof, which consists of the following components in parts by weight: 3.7 parts of lidocaine hydrochloride, 3 parts of xanthan gum, 2 parts of tragacanth gum, carboxymethyl 2 parts of sodium cellulose base, 3 parts of 0.1M sodium hydroxide, 100 parts of water for injection, 1.8 parts of vitamin P, 2.5 parts of acacia flower oil, 2.5 parts of eucalyptus oil, 3 parts of Span 65 and 2 parts of Tween 80 , 3.5 parts polyethylene glycol 400.
[0051] A preparation method for lidocaine hydrochloride gel, comprising the following steps:
[0052] a, extracting acacia flower oil and eucalyptus oil;
[0053] B, acacia flower oil and eucalyptus oil that a step is extracted and Span 65 and Tween 80, Polyethylene Glycol 400 mix homogeneously;
[0054] c. Dissolve an appropriate amount of vitamin P in water for injection to obtain a vitamin P aqueous solution; then weigh an appropriate amount of gel ...
Embodiment 3
[0058] This embodiment relates to a lidocaine hydrochloride gel and a preparation method thereof, which consists of the following components in parts by weight: 2 parts of lidocaine hydrochloride, 1 part of xanthan gum, 1 part of methyl cellulose, carboxylate 1 part of sodium methylcellulose, 1.65 parts of 0.1M sodium hydroxide, 70 parts of water for injection; 1.2 parts of vitamin P; 1 part of eucalyptus oil, 1 part of acacia flower oil, 1.2 parts of Span 65 and 1.5 parts of Tween 80. 2 copies of Xitu Mage.
[0059] A preparation method for lidocaine hydrochloride gel, comprising the following steps:
[0060] a, extract eucalyptus oil, acacia flower oil;
[0061] B, the eucalyptus oil that a step extracts, acacia oil are mixed with Span 65, part of Tween 80, and Situma Ge;
[0062] c. Dissolve an appropriate amount of vitamin P in water for injection to obtain a vitamin P aqueous solution; then weigh an appropriate amount of gel matrix and add vitamin P aqueous solution to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com