Composite polypeptide calcium powder and preparation method and application thereof
A technology of peptide calcium powder and peptide calcium, applied in application, drug combination, pharmaceutical formula, etc., can solve the problems of calcium loss, lack of consideration of bone collagen synthesis, and poor calcium supplementation effect, so as to increase bone density and strengthen the body Immunity, bioavailability-enhancing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
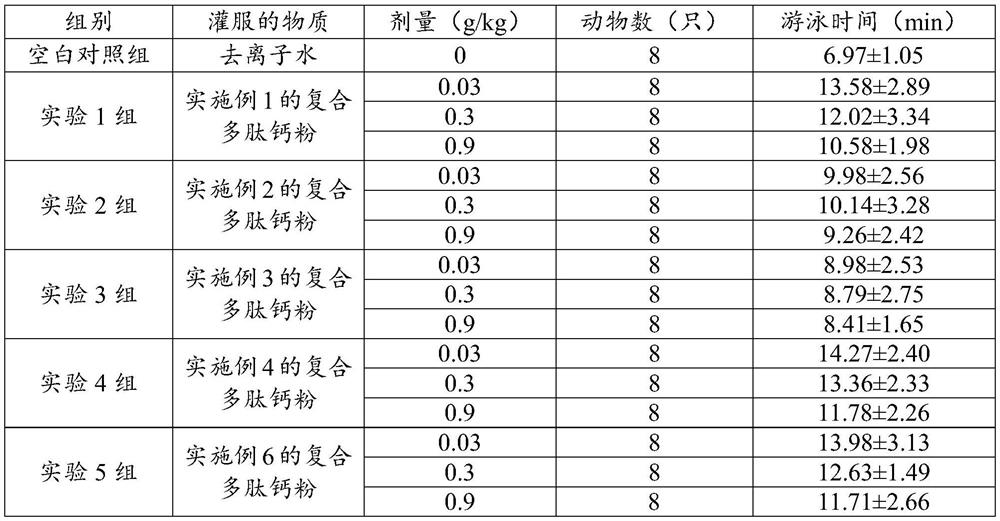
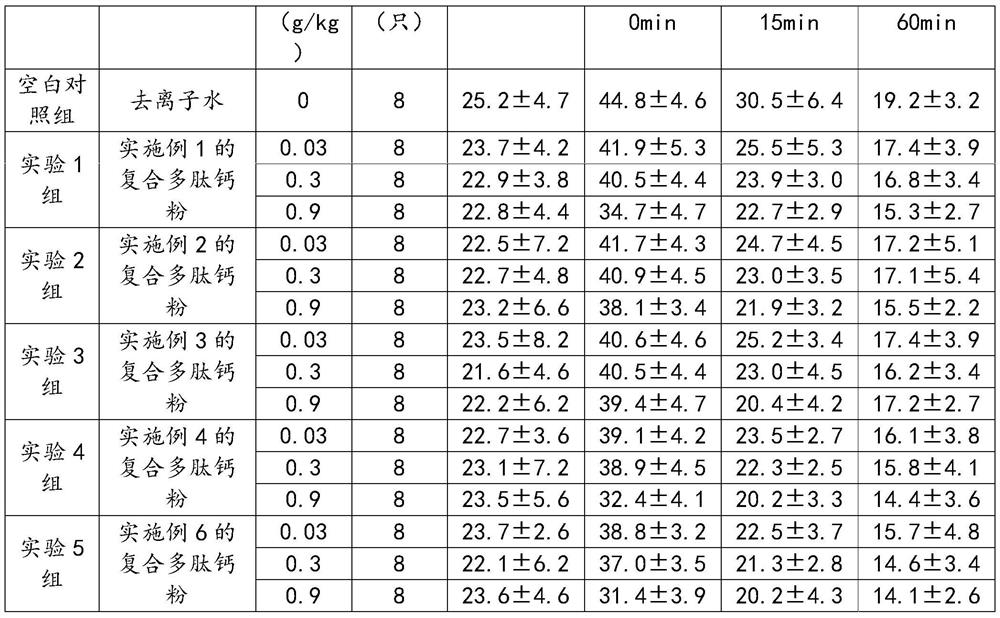
Examples
Embodiment 1
[0030] A compound polypeptide calcium powder comprises the following raw materials in parts by weight: 25 parts of bone collagen peptide, 20 parts of soybean peptide, 10 parts of sea cucumber peptide, 5 parts of oyster peptide, 4 parts of ginseng peptide, 20 parts of maltodextrin, and 10 parts of fruit powder , 6 parts of calcium aspartate and 5 parts of casein phosphopeptide, the preparation method comprises the following steps:
[0031] S1, ingredients: weigh 25 parts of bone collagen peptide, 20 parts of soybean peptide, 10 parts of sea cucumber peptide, 5 parts of oyster peptide, 4 parts of ginseng peptide, 20 parts of maltodextrin, 10 parts of fruit powder, 6 parts of calcium aspartate and 5 parts of casein phosphopeptide, set aside;
[0032] Bacterial inspection: carry out bacterial inspection on the main raw materials and auxiliary materials separately, and control the microbial indicators of raw and auxiliary materials within the qualified range;
[0033] S2, sieving:...
Embodiment 2
[0037] A composite polypeptide calcium powder comprises the following raw materials in parts by weight: 20 parts of bone collagen peptide, 10 parts of soybean peptide, 3 parts of sea cucumber peptide, 3 parts of oyster peptide, 2 parts of ginseng peptide, 15 parts of maltodextrin, and 5 parts of fruit powder , 5 parts of calcium aspartate and 3 parts of casein phosphopeptide. The preparation method steps are as follows:
[0038] S1, ingredients: weigh 20 parts of bone collagen peptide, 10 parts of soybean peptide, 3 parts of sea cucumber peptide, 3 parts of oyster peptide, 2 parts of ginseng peptide, 15 parts of maltodextrin, 5 parts of fruit powder, 5 parts of calcium aspartate and 3 parts of casein phosphopeptide, set aside;
[0039] Bacterial inspection: carry out bacterial inspection on the main raw materials and auxiliary materials separately, and control the microbial indicators of raw and auxiliary materials within the qualified range;
[0040] S2, sieving: check whet...
Embodiment 3
[0044] A composite polypeptide calcium powder comprises the following raw materials in parts by weight: 20 parts of bone collagen peptide, 10 parts of soybean peptide, 3 parts of sea cucumber peptide, 3 parts of oyster peptide, 2 parts of ginseng peptide, 15 parts of maltodextrin, and 5 parts of fruit powder , 5 parts of calcium aspartate and 3 parts of casein phosphopeptide. The preparation method steps are as follows:
[0045] S1, ingredients: weigh 20 parts of bone collagen peptide, 10 parts of soybean peptide, 3 parts of sea cucumber peptide, 3 parts of oyster peptide, 2 parts of ginseng peptide, 15 parts of maltodextrin, 5 parts of fruit powder, 5 parts of calcium aspartate and 3 parts of casein phosphopeptide, set aside;
[0046] Bacterial inspection: carry out bacterial inspection on the main raw materials and auxiliary materials separately, and control the microbial indicators of raw and auxiliary materials within the qualified range;
[0047] S2, sieving: check whet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com