Hyaluronic acid chemiluminescence immunoassay kit and preparation method thereof
A detection kit, the technology of uric acid chemistry, applied in chemiluminescence/bioluminescence, analysis by chemical reaction of materials, measurement devices, etc., can solve the problems of low performance such as sensitivity and linear range, and reduce manual operation. The effect of error, large specific surface area and small systematic error
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
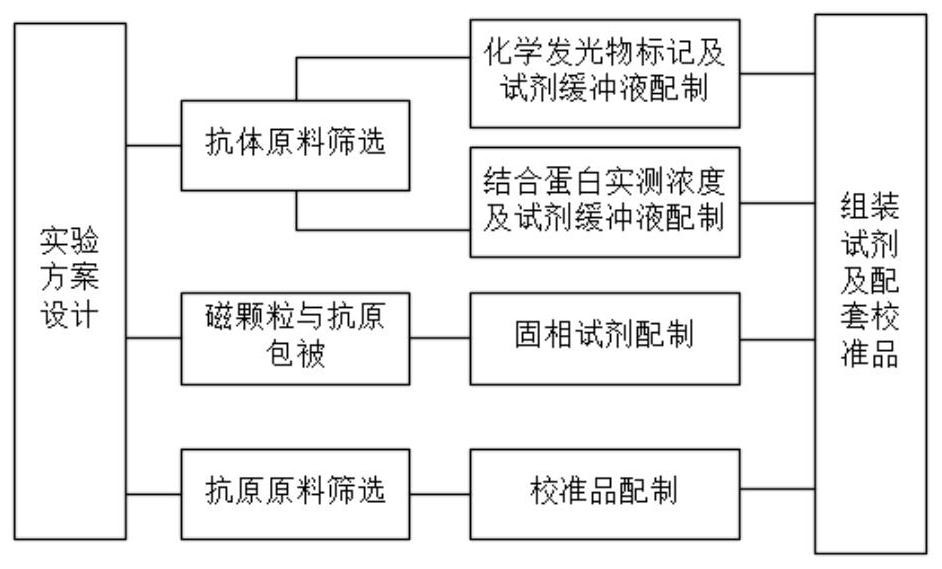
[0055] figure 1 It is a design diagram of the preparation process of the hyaluronic acid chemiluminescent immunoassay kit of the present invention, including: screening of antibody raw materials: preparation of chemiluminescence labeling and reagent buffer; magnetic particle and antigen coating, preparation of solid-phase reagents; antigen raw materials Screening, preparation of calibrators, final assembly of reagents and supporting calibrators. The preparation method of the hyaluronic acid chemiluminescence immunoassay kit provided by the present invention specifically comprises the following steps:
[0056] Step 1, preparation of R1 reagent:
[0057] After the replacement of the hyaluronic acid antigen, suspend the magnetic particles and the hyaluronic acid antigen overnight, add a blocking agent, collect the magnetic field, and take the supernatant to obtain 1% to 3% coated magnetic particles of the hyaluronic acid antigen; 100-500mM Bistris, 0.05%-0.1% Tween-20, 0.05%-0....
Embodiment 1
[0064] Embodiment 1: the preparation of hyaluronic acid chemiluminescent immunoassay kit:
[0065] Preparation of R1 reagent: After replacing the hyaluronic acid antigen, suspend the Tosyl magnetic beads and the hyaluronic acid antigen overnight, add a blocking agent, collect the supernatant, and obtain the Tosyl magnetic beads coated with 2% hyaluronic acid antigen. particles. In 100mM Bistris, 0.05% Tween-20, 0.05% Proclin300, pH 6.0 buffer, the R1 reagent with a magnetic bead concentration of 0.072% was prepared.
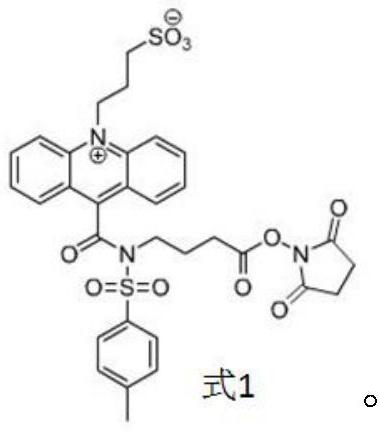
[0066] Preparation of R2 reagent: put 500 μg hyaluronic acid-binding protein monoclonal antibody into a centrifuge tube, make sure that the antibody is at the bottom of the centrifuge tube, add carbonic acid buffer solution, mix well, add acridinium ester DMF solution after mixing, follow The labeling molar ratio of hyaluronan-binding protein monoclonal antibody to chemiluminescent marker is 1:10 for labeling, and the hyaluronan-binding protein chemiluminescent ...
Embodiment 2
[0068] Embodiment 2: the preparation of hyaluronic acid chemiluminescent immunoassay kit:
[0069] Preparation of R1 reagent: After replacing the hyaluronic acid antigen, suspend the Tosyl magnetic beads and the hyaluronic acid antigen overnight, add a blocking agent, collect the supernatant, and obtain the Tosyl magnetic beads coated with 3% hyaluronic acid antigen. particles. In 500mM Bistris, 0.1% Tween-20, 0.1% Proclin300, pH 6.5 buffer, the R1 reagent with a magnetic bead concentration of 0.05% was prepared.
[0070] Preparation of R2 reagent: put 500 μg hyaluronic acid-binding protein monoclonal antibody into a centrifuge tube, make sure that the antibody is at the bottom of the centrifuge tube, add carbonic acid buffer solution, mix well, add acridinium ester DMF solution after mixing, follow The labeling molar ratio of hyaluronan-binding protein monoclonal antibody to chemiluminescent marker is 1:3 for labeling, and the hyaluronan-binding protein chemiluminescent mark...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com