Method for efficiently and sequentially inducing macrophages to be polarized in vitro
A macrophage and sequential technology, applied in the field of efficient sequential induction of macrophage polarization in vitro, can solve the problems of insignificant differences in protein expression, uncertain macrophage status, and inability to accurately differentiate THP-1 cells. Achieve accurate research and eliminate the effect of macrophage polarization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the sequential induction method of the present invention
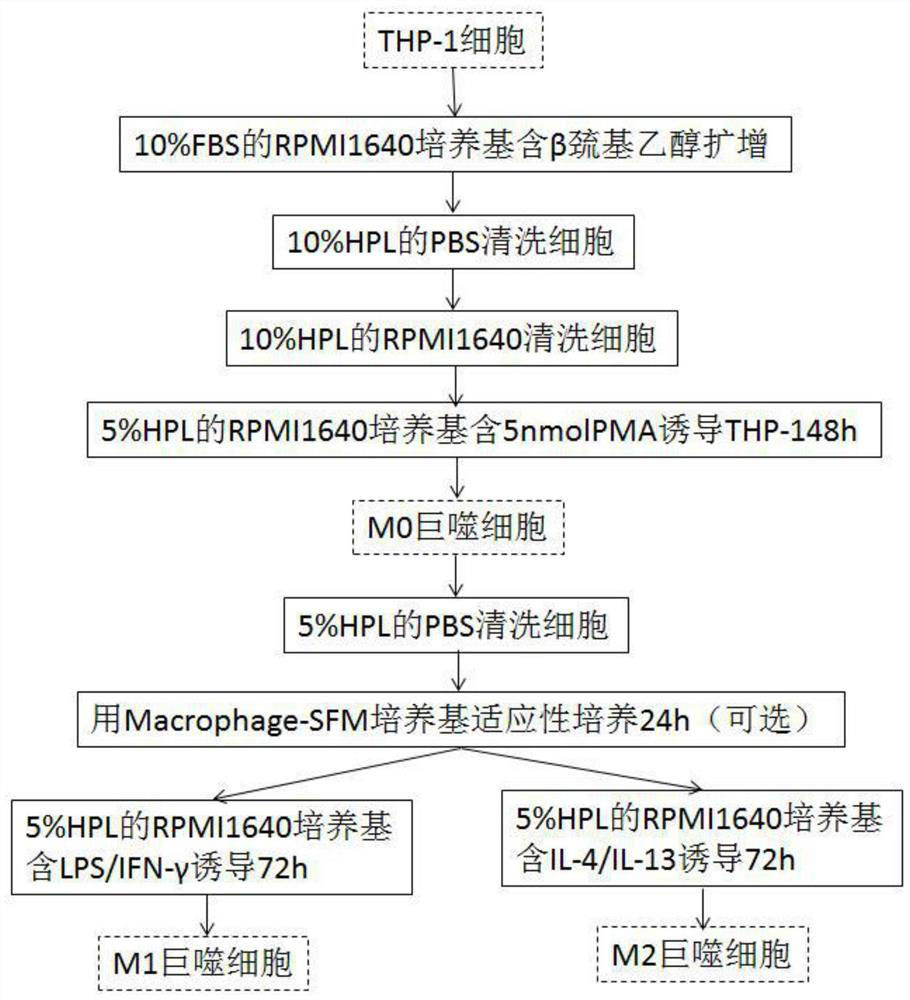
[0031] see figure 1 , the embodiment of the present invention is a method for efficiently and sequentially inducing the polarization of macrophages in vitro, comprising the following steps:
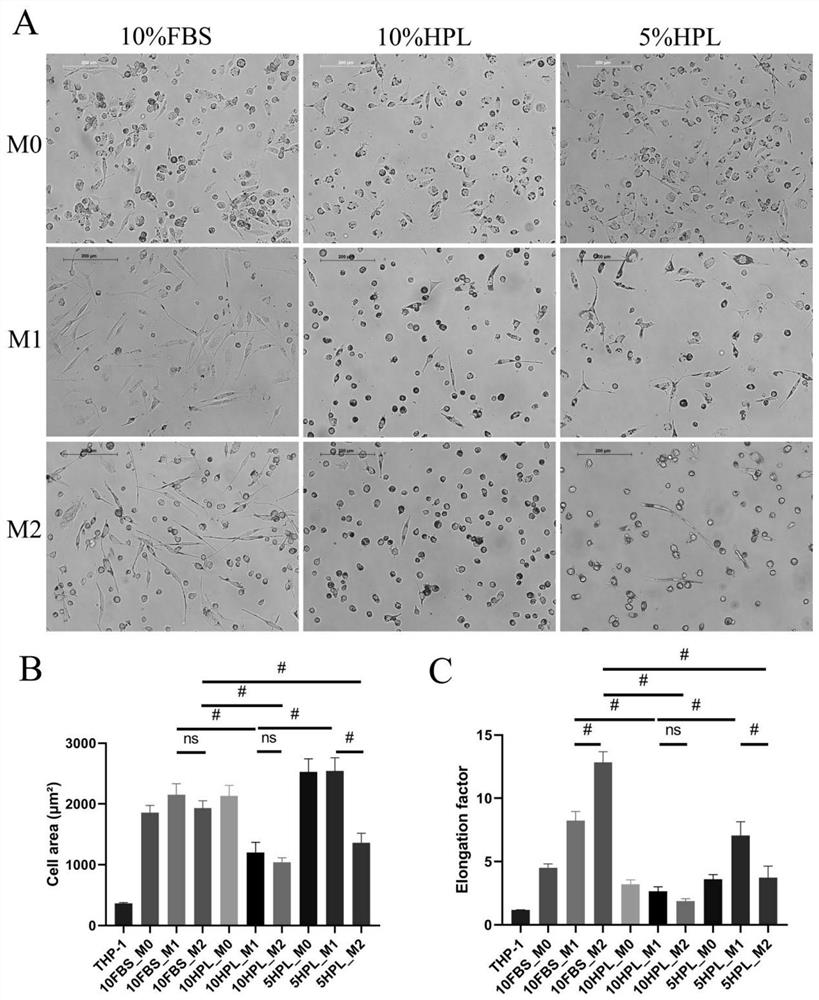
[0032] S1 expansion and proliferation culture: THP-1 cells are expanded and proliferated. Specifically, THP-1 cells were routinely recovered and subcultured using RPMI1640 medium containing 10% FBS and 0.05 nM β-mercaptoethanol to ensure the activity and high proliferation of THP-1 cells in an undifferentiated state. In the expansion and proliferation culture stage of THP-1 cells, avoid using human HPL for cell culture, if human HPL is used, it will cause abnormal adhesion of THP-1 cells.
[0033] In this example, THP-1 cells were purchased from the American Type Culture Collection (ATCC) Corporation, and cryopreserved in liquid nitrogen until use. RPMI 1640 medium containing 10% heat-inactivated FBS, 1% pe...
Embodiment 2
[0043] Embodiment 2: analysis verification
[0044] 1. Materials and methods
[0045] 1. THP-1 cell culture
[0046] THP-1 cells were purchased from the American Type Culture Collection (ATCC) and kept cryopreserved in liquid nitrogen until use. RPMI 1640 medium containing 10% heat-inactivated FBS, 1% penicillin / streptomycin, 1% amphotericin B and 0.05 nM 2-mercaptoethanol was used to expand THP-1 cells. Place cells at 75cm 2 flasks and cultured in 5% CO 2 , 37 ℃ humid incubator. THP-1 cells were centrifuged at 1400 rpm for 5 min at 8°C for cell passage. THP-1 cells at passage 16 were used for macrophage induction experiments.
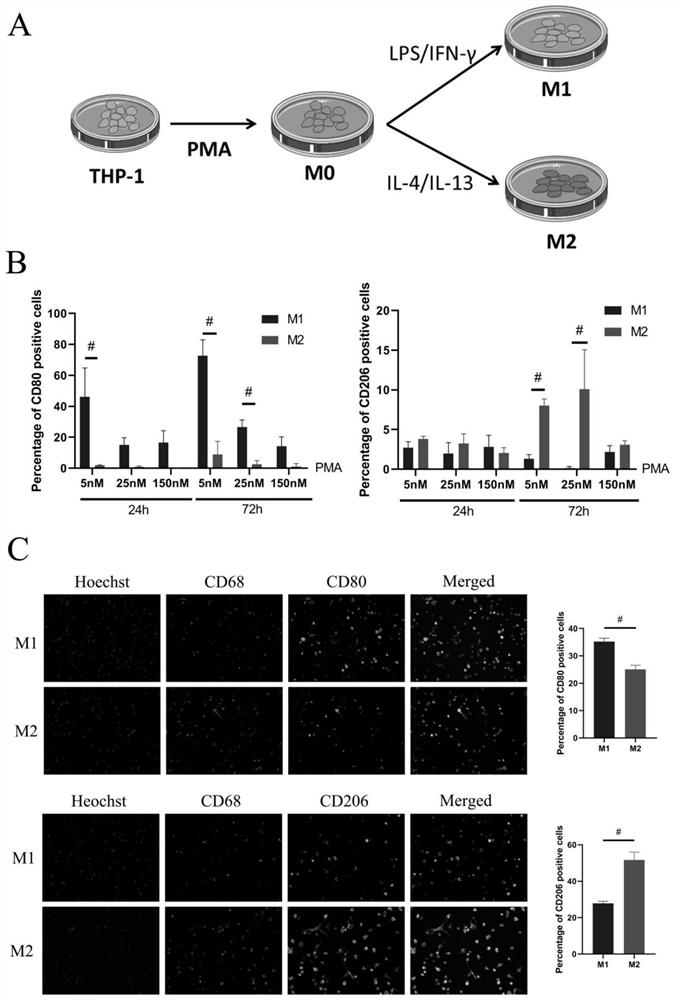
[0047] 2. Comparison of PMA concentration and subsequent cytokine induction time during THP-1 macrophage differentiation
[0048] THP-1 cells were induced to differentiate into M0 macrophages using PMA. will be 6×10 5 THP-1 cells were resuspended in 10% FBS RPMI1640 medium containing different concentrations of PMA (5nM, 25nM, 150nM), and then...
Embodiment 3
[0068] Embodiment 3: application experiment
[0069] Experiment of co-cultivating periosteal mesenchymal stem cells and M1 macrophages: Mesenchymal stem cells have a very powerful immunoregulatory effect and can strongly inhibit the inflammatory response. The applicant found that when the jaw bone periosteum stem cells were co-cultured with the above-mentioned THP-1 M1 macrophages, the jaw bone periosteum mesenchymal stem cells showed reduced expression of CD80 and CD86 (inflammation-related proteins) in M1 type macrophages ( Image 6 ), the expression changes of related proteins indicate that periosteal mesenchymal stem cells can reduce the polarization of M0 to M1 macrophages, and have a tendency to polarize to M2 macrophages. This shows that the macrophages obtained by the above scheme can be effectively used in the study of the secretion regulation of mesenchymal stem cells in the co-culture system; similarly, they can also be used for M0 / M1 / M2 macrophages to regulate the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com