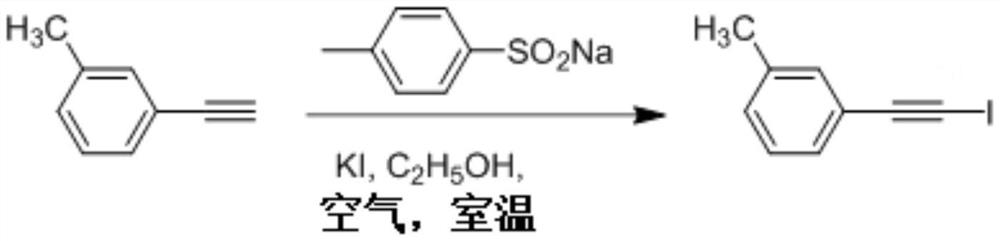
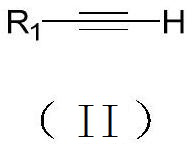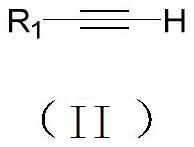Synthesis method of 1-iodo-alkyne compound
A synthetic method and compound technology, applied in the preparation of organic compounds, silicon organic compounds, organic chemical methods, etc., to achieve the effects of easy products, wide adaptability of substrates, and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038] Add 0.6mmol KI, 0.75mmol sodium p-toluene sulfinate, 4mL ethanol and 0.5mmol phenylacetylene to an ordinary test tube sequentially, open the test tube to the air, and stir the reaction at room temperature for about 12 hours. Followed by TLC to complete the reaction, separated by column chromatography to obtain a yellow oil, the eluent was sherwood oil / ethyl acetate (volume ratio of 50:1), the structure of the compound was obtained by NMR 1 H NMR, 13 C NMR and mass spectrometric tests. Yellow liquid, isolated yield 93%;
[0039] 1 H NMR (400MHz, CDCl 3 )δ7.44-7.42(m,2H),7.35-7.28(m,3H); 13 C NMR (100MHz, CDCl 3 )δ132.34,128.79,128.58,128.23,127.40,123.39,94.14,6.04; Ms(ESI):229[M+H] + .
Embodiment 2
[0041]
[0042] Add 0.6mmol KI, 0.75mmol sodium p-toluene sulfinate, 4mL ethanol and 0.5mmol p-toluene acetylene to an ordinary test tube in sequence, open the test tube to the air, and stir and react at room temperature for 12 hours. Followed by TLC to complete the reaction, separated by column chromatography to obtain a yellow oil, the eluent was sherwood oil / ethyl acetate (volume ratio of 50:1), the structure of the compound was obtained by NMR 1 H NMR, 13 C NMR and mass spectrometric tests. Yellow liquid, isolated yield 86%;
[0043] 1 H NMR (400MHz, CDCl 3 )δ7.32(d, J=8.1Hz, 2H), 7.11(d, J=7.9Hz, 2H), 2.35(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ139.02, 132.20, 129.25, 128.98, 127.34, 120.37, 94.25, 21.52, 4.84; Ms(ESI):243[M+H] + .
Embodiment 3
[0045]
[0046] 0.6mmol KI, 0.75mmol sodium p-toluene sulfinate, 4mL ethanol and 0.5mmol p-ethylphenylacetylene were sequentially added into an ordinary test tube, and the test tube was exposed to the air, and stirred and reacted at room temperature for 12 hours. Followed by TLC to complete the reaction, separated by column chromatography to obtain a yellow oil, the eluent was sherwood oil / ethyl acetate (volume ratio of 50:1), the structure of the compound was obtained by NMR 1 H NMR, 13 C NMR and mass spectrometric tests. Yellow liquid, isolated yield 82%;
[0047] 1 H NMR (400MHz, CDCl 3)δ7.34(d, J=8.1Hz, 2H), 7.13(d, J=8.0Hz, 2H), 2.64(q, J=7.6Hz, 2H), 1.21(t, J=7.6Hz, 3H) ; 13 C NMR (100MHz, CDCl 3 )δ 145.29, 132.32, 132.31, 127.80, 120.61, 94.30, 28.85, 15.29, 4.90; Ms(ESI): 257[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com