Epalrestat sustained release preparation and preparation method thereof
A sustained-release preparation, the technology of epalrestat, is used in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. problems, to increase compliance, avoid drug burst effects, and reduce the incidence of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
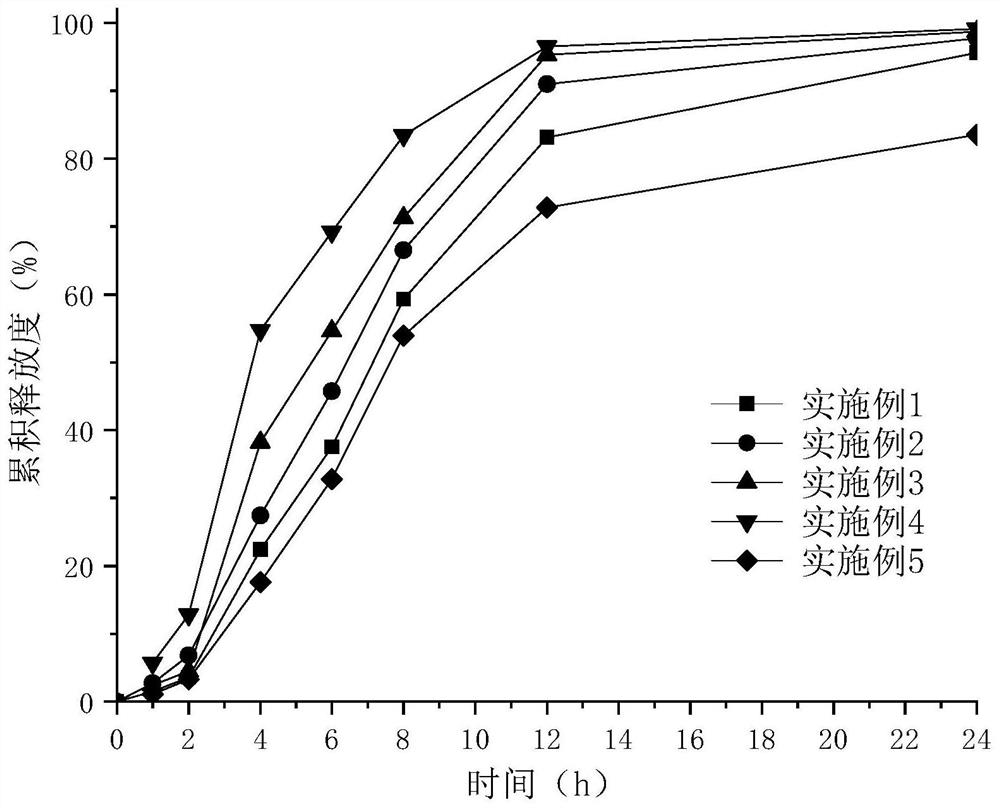
Examples
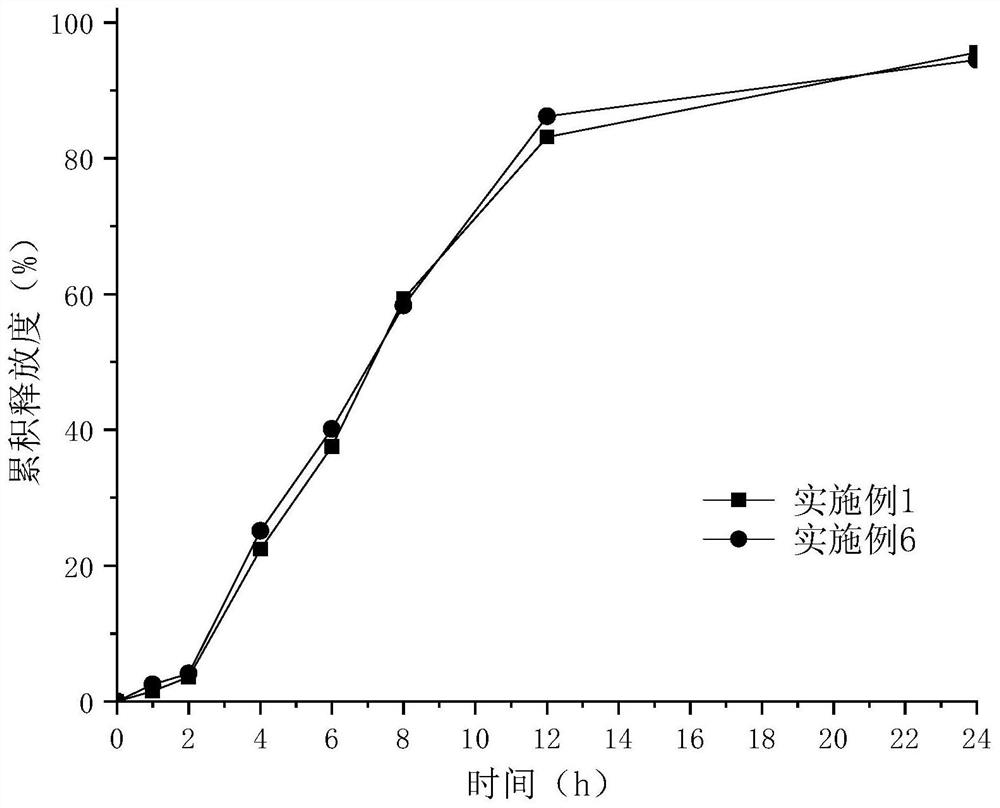
Embodiment 1
[0039] (1) Drug-containing sustained-release pellet core (per 1000 capsules)
[0040]
[0041]
[0042] (2) Isolation coating layer (per 1000 capsules)
[0043] Opadry YS-1-7003 17.1g
[0044] Purified water 154g
[0045] The coating weight gain was about 3%.
[0046] (3) Enteric coating layer (per 1000 capsules)
[0047] Sureteric 58.56g
[0048] Antifoam emulsion 0.19g
[0049] Purified water 331g
[0050] The coating weight gain was about 10%.
[0051] (4) Protective coating layer (per 1000 capsules)
[0052] Opadry YS-1-7003 19.3g
[0053] Purified water 174g
[0054] The coating weight gain was about 3%.
Embodiment 1
[0055] Embodiment 1——Example 10 Epalrestat sustained-release preparation preparation process:
[0056] The epalrestat raw material is micronized, particle size D 90 About 50μm, pass the auxiliary material through a 40-mesh sieve;
[0057] Preparation of soft material: weigh epalrestat, filler, release regulator, and sustained-release framework material according to the prescription amount, place them in a wet granulator and mix them evenly, spray an appropriate amount of binder aqueous solution with a certain concentration, and open Stirring knife for preparing soft materials;
[0058] Preparation of pellets: put the above-mentioned soft materials in an extrusion spheronizer to prepare pellets. Set the sieve aperture to 0.8 mm, the extrusion speed to 10-25 rpm, the rounding speed to 500-1700 rpm, and the rounding time to 2-5 minutes. Sieve, take 18-40 mesh pellets, and dry in a fluidized bed, set the drying temperature at 60°C, and dry for 20-25 minutes;
[0059] Fluidized...
Embodiment 2
[0066] Drug-containing sustained-release pellet core (per 1000 capsules)
[0067]
[0068] Hypromellose E5 (release modifier) 15g
[0069] Lactose G200 (filler) 90g
[0070] Microcrystalline Cellulose PH101 (Filler) 90g
[0071] Povidone K30 (adhesive) 8g
[0072] Appropriate amount of purified water
[0073] Other auxiliary materials category and dosage and preparation process are all the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com