Metal-air battery based on Fe(II)/Fe(III) chemical oxidation and electrochemical reduction
A metal-air battery and chemical oxidation technology, which is applied in the direction of fuel cell type half cell and secondary cell type half cell, etc., can solve the problems of catalytic activity not as good as noble metal catalyst, difficult to maintain catalytic activity for a long time, poor stability of noble metal catalyst, etc. , to achieve fast diffusion speed and electrochemical reduction reaction speed, realize uninterrupted and stable power supply, and enhance the effect of continuous discharge capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
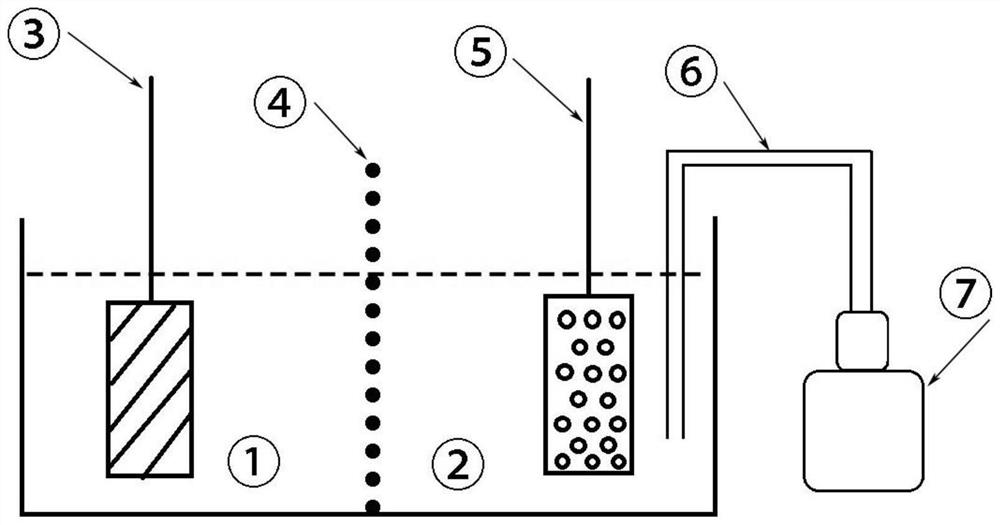
[0043] Immerse the industrial pure aluminum metal plate in the degassed saturated sodium chloride solution to obtain the negative electrode of the battery;
[0044] Immerse the graphite felt electrode in 0.5mol / L acid ferric nitrate solution to obtain the positive electrode of the battery;
[0045] The negative electrode electrolyte and the positive electrode electrolyte are separated by an anion semi-permeable membrane;
[0046] One end of the vent tube is immersed in the positive electrolyte, and the other end is connected to an air pump to form a metal-air battery based on Fe(II) / Fe(III) chemical oxidation and electrochemical reduction.
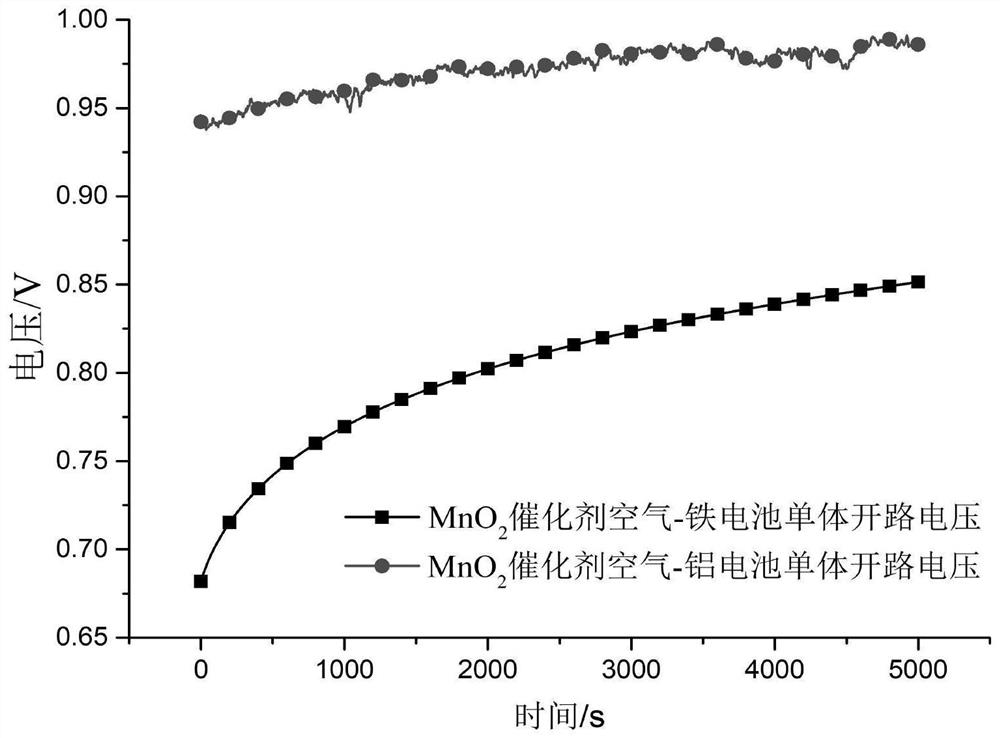
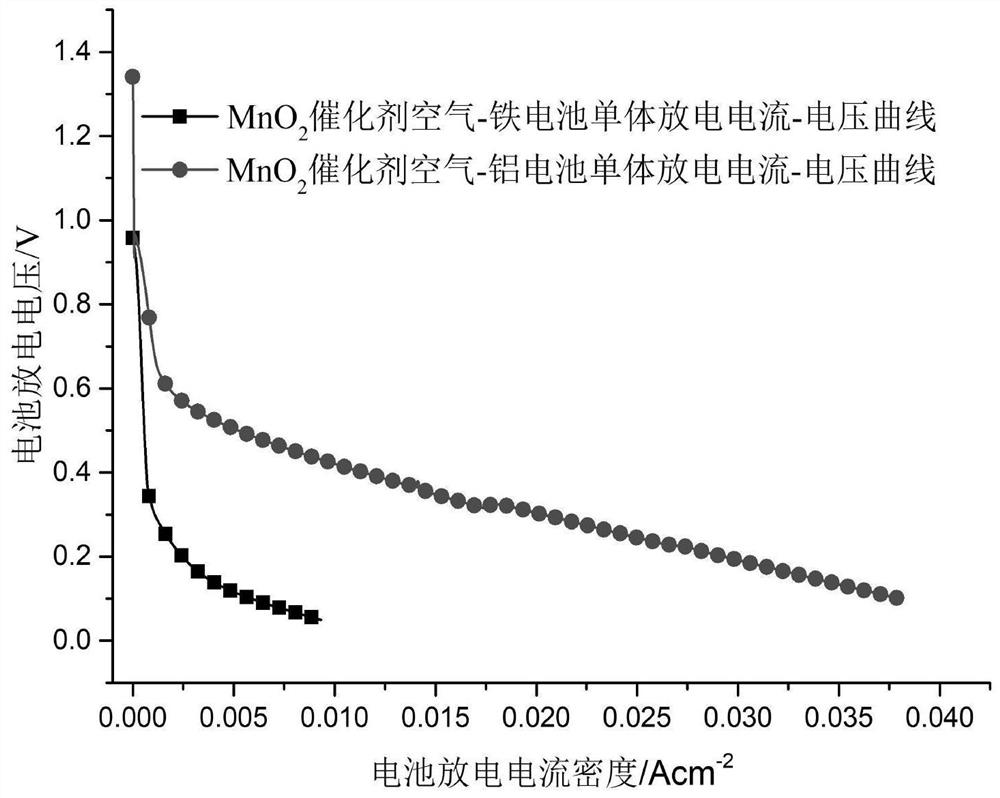
[0047] Test the discharge performance of the battery, the open circuit voltage is 1.33V, and the maximum discharge current density is 70mA×cm -2 .
Embodiment 2
[0049] Immerse the industrial pure aluminum metal plate in the degassed 3mol / L sodium chloride solution to obtain the negative electrode of the battery;
[0050] Immerse the graphite felt electrode in 1mol / L acid ferric sulfate solution to obtain the positive electrode of the battery;
[0051] The negative electrode electrolyte and the positive electrode electrolyte are separated by an anion semi-permeable membrane;
[0052] One end of the vent tube is immersed in the positive electrolyte, and the other end is connected to an air pump to form a metal-air battery based on Fe(II) / Fe(III) chemical oxidation and electrochemical reduction.
[0053] Test the discharge performance of the battery, the open circuit voltage is 1.41V, and the maximum discharge current density is 80mA×cm -2 .
Embodiment 3
[0055] Immerse the industrial pure aluminum metal plate in the degassed 2mol / L sodium chloride solution to obtain the negative electrode of the battery;
[0056] Immerse the graphite felt electrode in 2mol / L acid ferric chloride solution to obtain the positive electrode of the battery;
[0057] The negative electrode electrolyte and the positive electrode electrolyte are separated by an anion semi-permeable membrane;
[0058] One end of the vent tube is immersed in the positive electrolyte, and the other end is connected to an air pump to form a metal-air battery based on Fe(II) / Fe(III) chemical oxidation and electrochemical reduction.
[0059] Test the discharge performance of the battery, the open circuit voltage is 1.41V, and the maximum discharge current density is 80mA×cm -2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com