Agent for improving cytokine release syndrome, etc.
A technology of cytokines and syndromes, applied in the direction of anti-inflammatory agents, drug combinations, non-central analgesics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
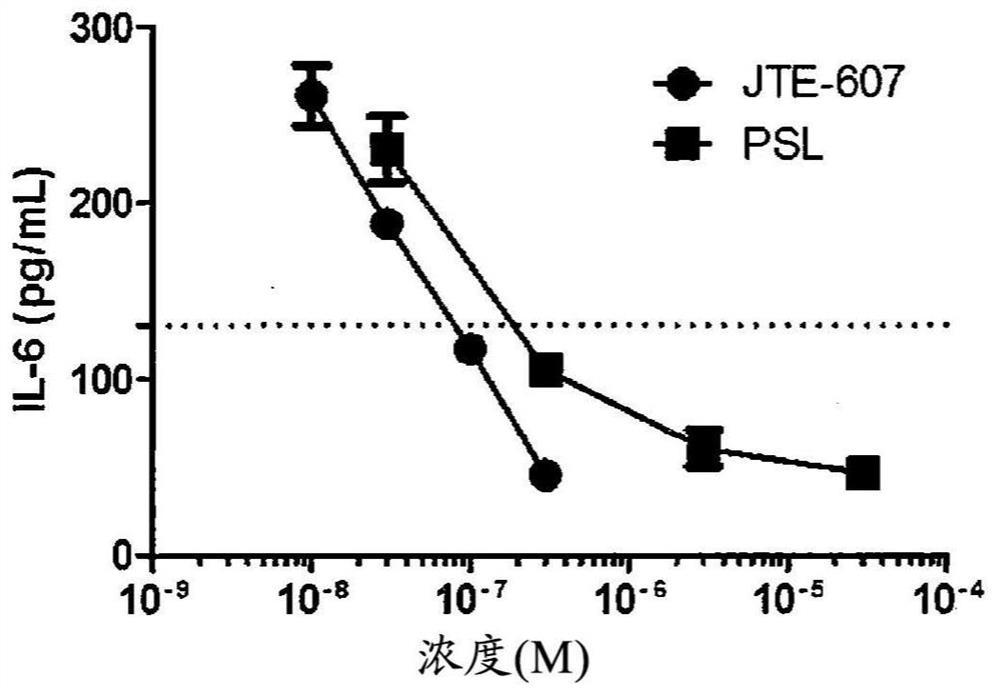
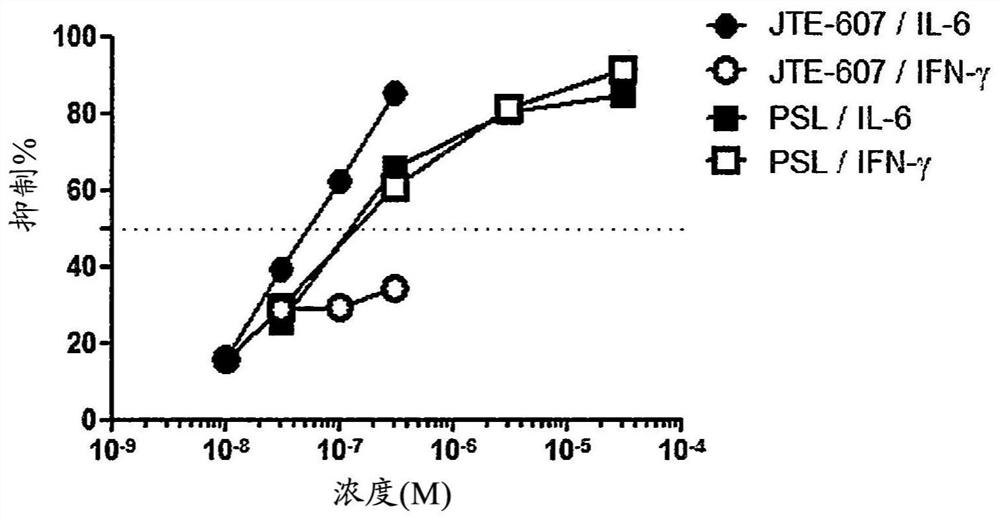
Image
Examples
Embodiment 1
[0210] 1. Method
[0211] 1.1 Materials
[0212] 1.1.1 Cells
[0213] Cryopreserved Human PBMC (PBMC): BIOPREDIC
[0214] CD19-CAR-T cells (CAR-T cells) containing CD3 / CD28 signal sequences: provided by the Molecular Therapy Field, Institute of Medical Science, The University of Tokyo
[0215] CD19-expressing K562 cell line (target cell): Provided by the Molecular Therapy Field of the Institute of Medical Sciences, The University of Tokyo
[0216] Human Monocyte (monocyte): BIOPREDIC
[0217] Lenti-X293T cells: Clontech
[0218] 1.1.2 Reagents
[0219] RPMI1640 medium: Thermo fisher (Gibco)
[0220] Fetal bovine serum (FBS): Thermo fisher (Gibco)
[0221] D-PBS(-): Wako Pure Chemical Industries
[0222] Ethylenediaminetetraacetic Acid Disodium Dihydrogen Dihydrate (EDTA): Pure Chemistry
[0223] Bovine Serum Albumin (BSA): SIGMA
[0224] Dimethyl sulfoxide (DMSO): SIGMA
[0225] Penicillin-Streptomycin-Glutamine: Thermo fisher
[0226] 2-Mercaptoethanol: SIGMA
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com