Fudosteine oral liquid and preparation method thereof
A technology of fudosteine oral liquid and stetan oral liquid, which is applied in the field of biomedicine, can solve the problems of increasing production cost, increasing the workload of preparation products, product quality, etc., to reduce conversion, improve dimensional stability, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
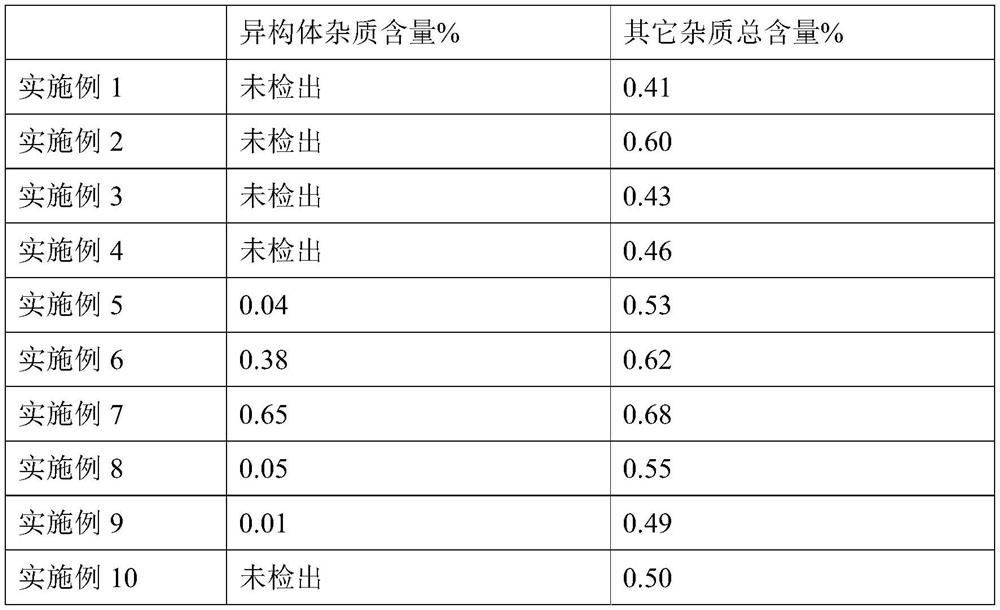
Examples
Embodiment 1
[0050] The present embodiment provides a kind of 500mL fudosteine oral liquid, described fudosteine oral liquid comprises 8% fudosteine, 3.6% zinc sulfate, acid-base regulator (1M citric acid 5.9mL, 1M citric acid sodium 4.1mL), 0.05% sodium benzoate, 10% trehalose, 0.01% lemon essence, 0.1% tartrazine and water; wherein the mol ratio of fudosteine and zinc sulfate is 2:1, the pH value of the oral solution is 4.0. Its preparation method is as follows:
[0051] (1) Mix zinc sulfate with 200mL water and stir until dissolved;
[0052] (2) Mix the solution obtained in step (1) with fudosteine, stir until dissolved, and add an acid-base regulator to adjust the pH value of the solution to 4.0;
[0053] (3) The solution obtained in step (2) was subjected to a chelation reaction at 50°C for 60 minutes, cooled, and then the remaining components were added to it. After mixing evenly, water was added to a total volume of 500 mL to obtain the fudosteine Oral solution.
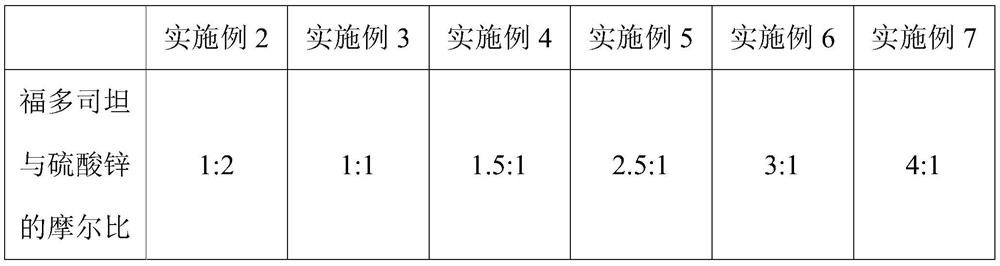
Embodiment 2-7
[0055] This embodiment provides six kinds of 500mL fudosteine oral liquid, and its difference with embodiment 1 is that the molar ratio of fudosteine and zinc sulfate is different, as shown in the following table:
[0056]
[0057] Its preparation method is identical with embodiment 1.
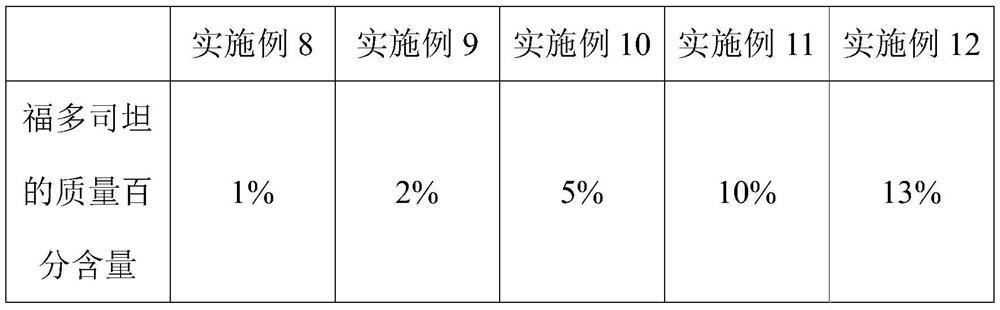
Embodiment 8-12
[0059] The present embodiment provides five kinds of 500mL fudosteine oral liquid, and its difference with embodiment 1 is only that the mass percentage content of fudosteine is different, and wherein the mol ratio of fudosteine and zinc sulfate is 2:1, As shown in the table below:
[0060]
[0061] Its preparation method is identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com