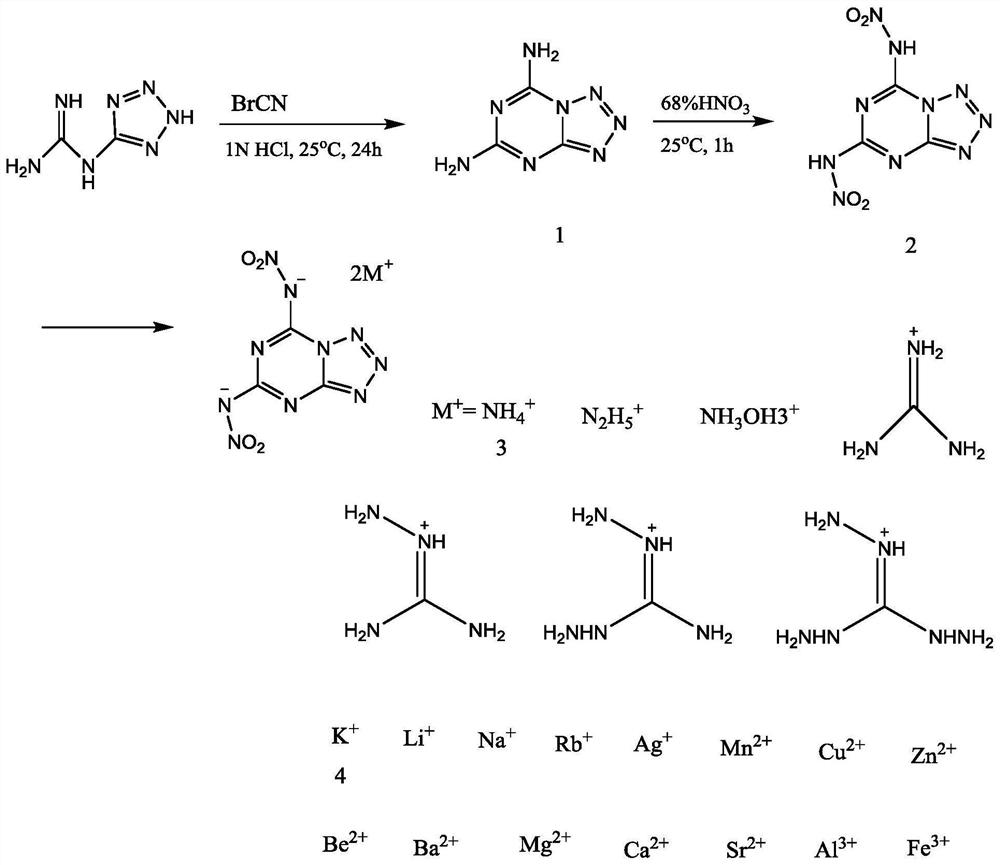
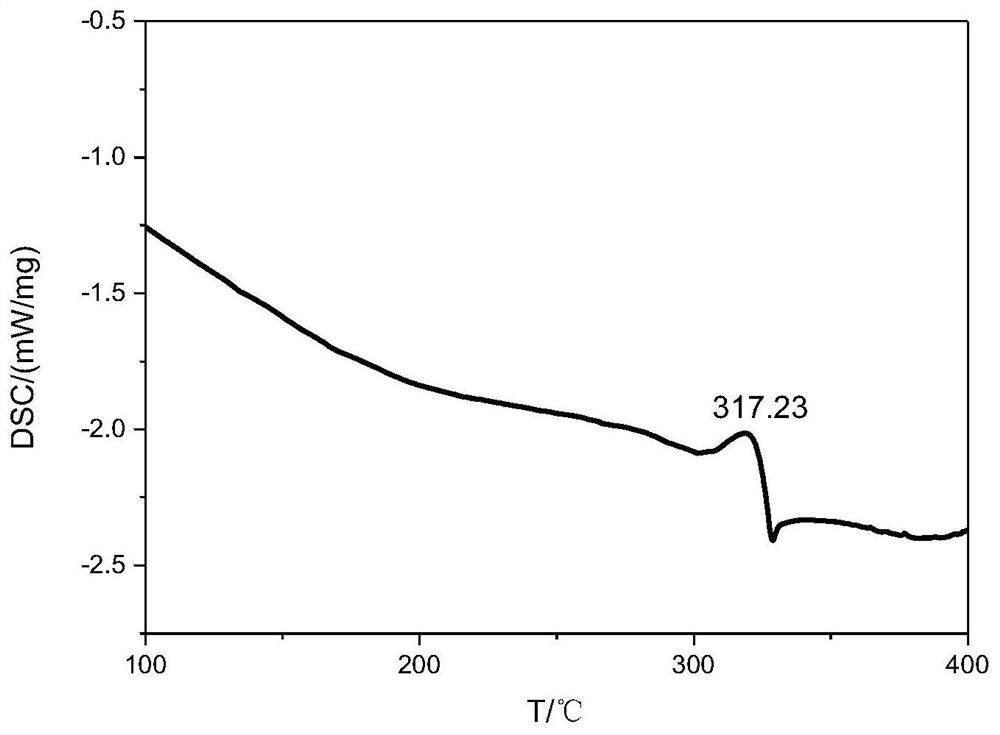
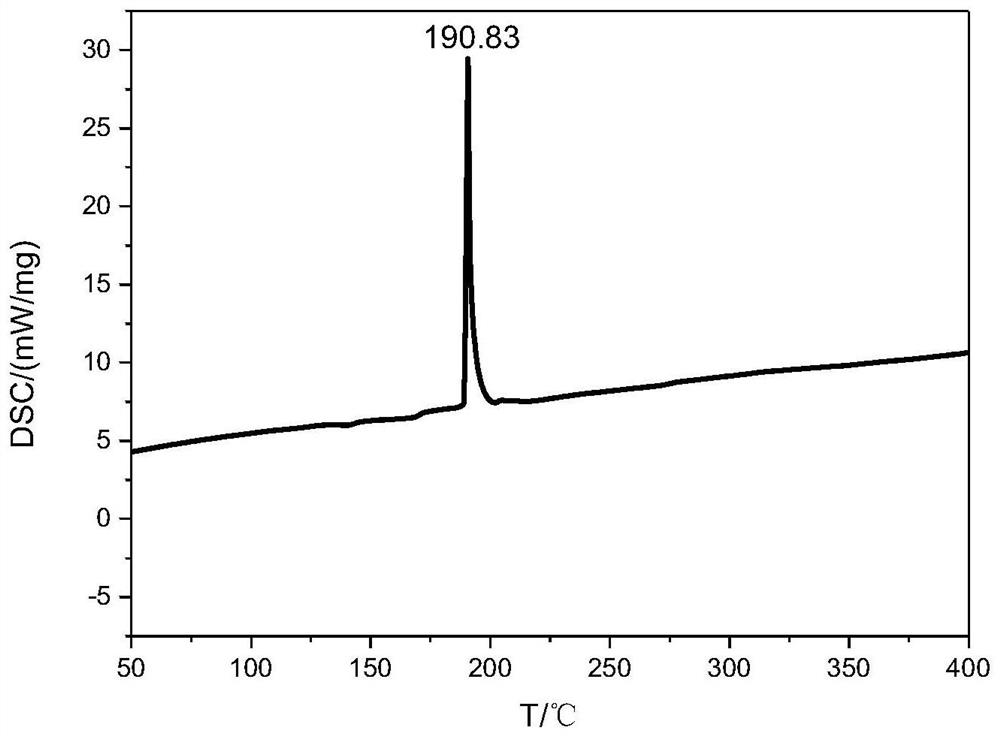
A nitrogen-rich condensed ring compound, its derivatives, and its preparation method
A compound, fused ring technology, applied in the direction of nitrated acyclic/alicyclic/heterocyclic amine explosive compositions, organic chemistry, etc. energy boosting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] A kind of preparation method of nitrogen-rich condensed ring compound of the present invention comprises the following steps:
[0040] Step 1: Add 0.25 to 5.08 grams of 1-(2H-tetrazol-5-yl)guanidine to 60ml of 0.5 to 2M hydrochloric acid in an ice-water bath at -5 to 5°C, and stir for 5 to 60 minutes to obtain a suspension , and then add BrCN in the amount of 1-(2H-tetrazol-5-yl) to the suspension, stir for 5 to 60 minutes, remove the ice-water bath, let it slowly return to room temperature, and continue to close the ring at this temperature React for 6-48 hours, hydrochloric acid is a catalyst and provides an acidic environment. After the reaction, cool the reaction liquid at 0-5°C, and then adjust the pH to 4-7 with 0.1-2M NaOH. At this time, a large amount of white precipitates are precipitated. Filter the obtained solid powder, rinse with deionized water, and dry. A white powder was obtained, which was denoted as compound 1, namely tetrazolo[1,5-a][1,3,5]-triazine-...
Embodiment 1
[0048] The specific synthetic steps of compound 1, compound 2, compound 3 and compound 4 are shown in figure 1 as shown,
[0049] Step 1: In an ice-water bath, add 2.54 grams (20 mmol) of 1-(2H-tetrazol-5-yl) guanidine to 60 ml of 1M HCl solution and stir for 20 minutes to obtain a suspension, then add 2.12 grams of ( 20 mmol) of BrCN was stirred for 10 minutes, the ice-water bath was removed, and it was slowly returned to room temperature, and the temperature was maintained to continue the reaction for 24 hours. After the reaction, the reaction solution was cooled in a refrigerator at 5°C for 12 hours, and the pH was adjusted to 7 with 1M NaOH. A large amount of white precipitates were precipitated. The obtained solid powder was filtered, rinsed with deionized water, and dried in air to obtain a white powder. Compound 1, tetrazolo[1,5-a][1,3,5]-triazine-5,7-diamine, yield 63.2%.
[0050] The obtained compound 1 was recrystallized with ethanol, filtered, and the filtrate was...
Embodiment 2
[0061] Step 1: In an ice-water bath, add 0.25 g of 1-(2H-tetrazol-5-yl)guanidine to 60 ml of 0.5M HCl solution and stir for 5 minutes to obtain a suspension, then add 1-(2H -Tetrazol-5-yl) BrCN with the same molar number of guanidine was stirred for 5 minutes, then the ice-water bath was removed, and it was slowly returned to room temperature, and the temperature was maintained to continue the reaction for 6 hours. After the reaction, the reaction solution was cooled in the refrigerator at 0°C for 12 hours, and the pH was adjusted to 4 with 1M NaOH. A large amount of white precipitates were precipitated. The resulting solid powder was filtered, rinsed with deionized water, and dried in air to obtain a white powder. Compound 1, tetrazolo[1,5-a][1,3,5]-triazine-5,7-diamine.
[0062] Step 2: In the ice-water bath, first add 3ml of concentrated nitric acid to the flask to cool it down. After 15 minutes, add 0.02 g of the compound 1 prepared above, stir and react for 10 minutes, ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com