Preparation method of polysubstituted bromomethyl benzo nitrogenous heterocyclic compound
An extraction and extraction device technology, applied in the field of pharmaceutical intermediate preparation, can solve the problems of inconvenient vibration operation of the extraction device, difficult implementation of the extraction process, small volume of the extraction device, etc., achieves good preparation effect, strong inventive concept and creativity, fixed strong enough effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
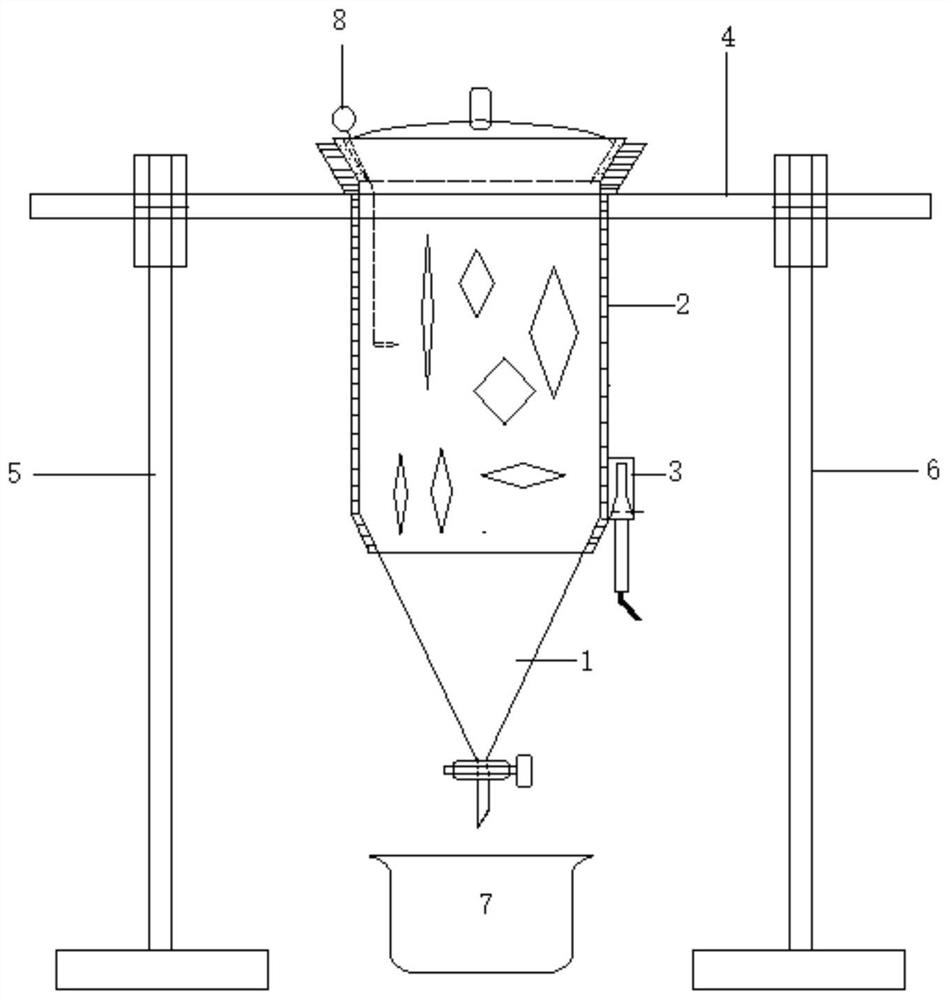
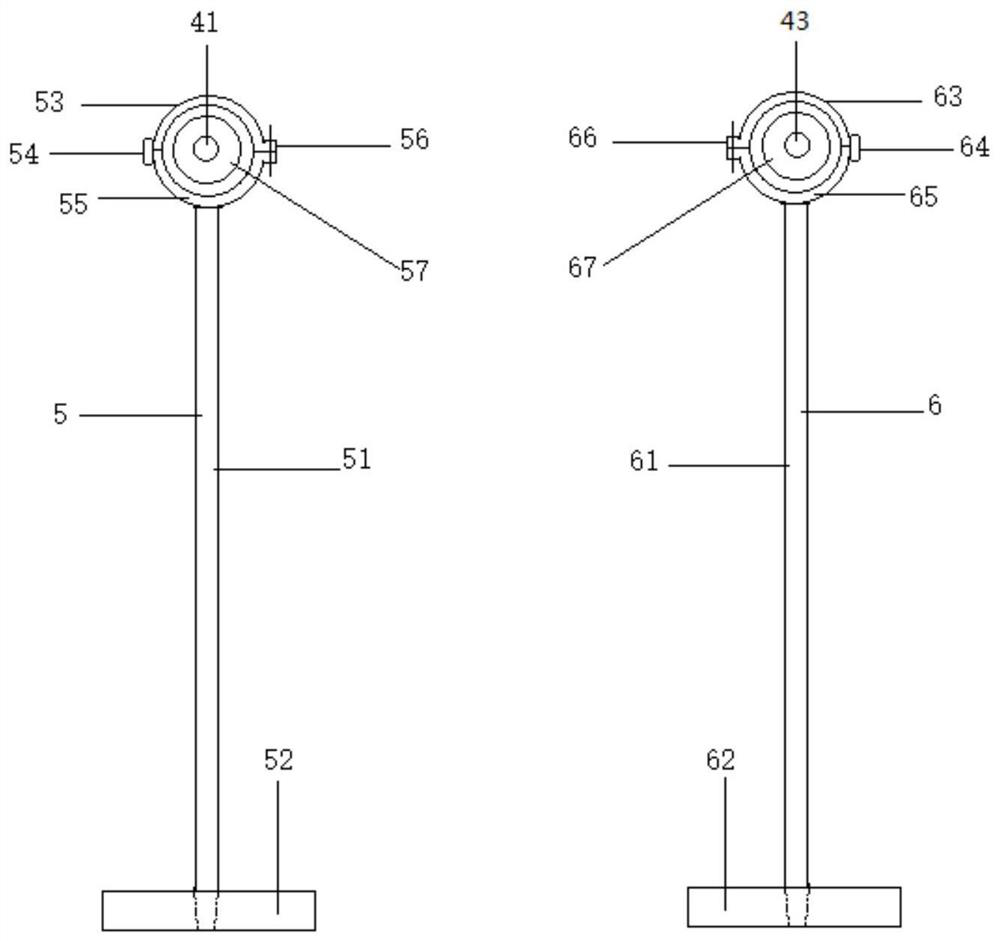
[0053] An ultrasonic extraction device is characterized by comprising an extraction part 1, a shell part 2, an ultrasonic part 3, a cross bar part 4, a left support part 5, a right support part 6, a glassware 7 and a demulsification component 8. The extraction device can be divided into multiple specifications, for example, the volume of the extraction part can be 1L, 2L, 3L, 4L, 5L to meet different requirements.
[0054] The extraction part 1 comprises a cylindrical part 11, an upper opening 12, an upper cover 13, a cone part 14, a liquid stopper group 15 and a liquid outlet 16. The main part of the extraction part 1 is spliced from top to bottom by an upper opening 12, a cylindrical part 11 and a conical part 14, the cylindrical part 11 is hollow, the upper opening 12 is larger and smaller, and the inner side is a frosted part 121; the outer edge of the upper cover 13 adapted to the frosted part 121 is a frosted surface; the upper center of the upper cover 13 is provided with a...
Embodiment 2
[0063] A preparation method of polysubstituted bromomethylbenzo nitrogen-containing heterocyclic compounds, which requires a large amount of extraction dose, is carried out by using an ultrasonic extraction device as described above, and is characterized by comprising the following steps.
[0064] (1) dissolve 64-68g acetone acetal in 1900-2100ml toluene, add 105-115g o-bromobenzylamine while stirring, raise the temperature to about 120℃, react until water division is finished, confirm that the reaction is complete through LCMS detection, and concentrate to obtain pale yellow liquid N-(2- bromophenyl)-1,1-dimethoxypropyl -2-.
[0065] (2) 165-175g of N-(2- bromophenyl)-1,1-dimethoxypropane -2- imine is dissolved in 1600-300ml of ethanol, the temperature is controlled to below 30-35 degrees Celsius, 55-59g of sodium borohydride is slowly added in batches, and after the addition is finished, the reaction is continued at 30-35 degrees Celsius for 8 hours. Add at least 1L of water to ...
Embodiment 3
[0071] A preparation method of polysubstituted bromomethylbenzo nitrogen-containing heterocyclic compounds, which requires a large amount of extraction dose, is carried out by using an ultrasonic extraction device as described above, and is characterized by comprising the following steps.
[0072] (1) 68g acetone acetal was dissolved in 2100ml toluene, 113.33g o-bromobenzylamine was added under stirring, the temperature was raised to about 120℃, and the reaction was completed. LCMS detection confirmed that the reaction was complete, and the light yellow liquid N-(2- bromophenyl)-1,1-dimethoxypropyl -2- imine was obtained by concentration. The yield is 100%.
[0073] (2) Dissolve 175g of N-(2- bromophenyl)-1,1-dimethoxypropyl -2- imine in 1800ml of ethanol, control the temperature to below 32℃, slowly add 59g of sodium borohydride in batches, keep the reaction at 32℃ for 10h after the addition, and confirm the reaction is complete by LCMS detection. Drop 300ml of water and concentr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Depth | aaaaa | aaaaa |
| Depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com