Superfine M-N-C non-noble metal carbon-based oxygen reduction catalyst, and preparation method and application thereof
A non-precious metal, catalyst technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of difficult to control the size and morphology of oxygen reduction catalysts of MOF materials, easy aggregation of metal active sites, and reduction of catalyst active site density.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
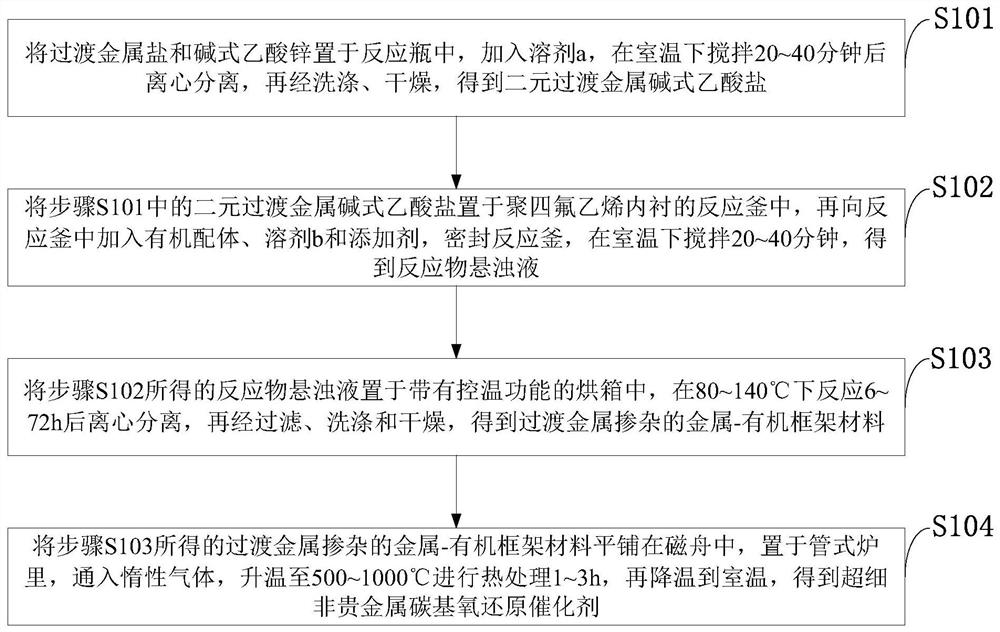
[0061] Such as figure 1 As shown, the preparation method of the ultrafine M-N-C non-noble metal carbon-based oxygen reduction catalyst provided by the invention comprises the following steps:
[0062] S101: Put transition metal salt and basic zinc acetate in a reaction bottle, add solvent a, stir at room temperature for 20-40 minutes, centrifuge, then wash and dry to obtain binary transition metal basic acetate;
[0063] S102: Place the binary transition metal basic acetate in step S101 in a polytetrafluoroethylene-lined reaction kettle, then add organic ligands, solvent b and additives to the reaction kettle, seal the reaction kettle, and set the temperature at room temperature Stir for 20 to 40 minutes to obtain a suspension of the reactant;
[0064] S103: Place the reactant suspension obtained in step S102 in an oven with temperature control function, react at 80-140°C for 6-72 hours, centrifuge, then filter, wash and dry to obtain transition metal-doped metal-organic fra...
Embodiment 1
[0082] The preparation method of the ultrafine M-N-C non-noble metal carbon-based oxygen reduction catalyst provided by the invention comprises the following steps:
[0083] (1) Put 20mL of cobalt nitrate aqueous solution (2M) and 100mg of basic zinc acetate in a reaction flask, add 1mL of ultrasonically dispersed ethanol, stir at room temperature for 30 minutes, and then centrifuge, wash with methanol, and place at 80°C Dry for 360min to obtain cobalt-doped basic zinc acetate;
[0084] (2) Place 30 mg of cobalt-doped basic zinc acetate in step (1) in a 20 mL polytetrafluoroethylene-lined reactor, and then add 100 mg of 2-methylimidazole, 5 mL of DMF, and 1 mL of Ionized water and 2mL triethylamine, seal the reaction kettle, stir at room temperature for 30 minutes to obtain a reactant suspension;
[0085] (3) Place the reactant suspension obtained in step (2) in an oven with temperature control function, react at 100°C for 12 hours, centrifuge, then filter, wash with ethanol,...
Embodiment 2
[0088] The preparation method of the ultrafine M-N-C non-noble metal carbon-based oxygen reduction catalyst provided by the invention comprises the following steps:
[0089] (1) Put 20mL of ferric nitrate aqueous solution (2M) and 100mg of basic zinc acetate into a reaction flask, add 1mL of ethanol after ultrasonic dispersion, stir at room temperature for 30 minutes, then centrifuge, wash with methanol, and place at 80°C Dry for 360min to obtain iron-doped basic zinc acetate;
[0090] (2) Place 30 mg of iron-doped basic zinc acetate in step (1) in a 20 mL polytetrafluoroethylene-lined reactor, and then add 100 mg of 2-methylimidazole, 5 mL of DMF, and 1 mL of Ionized water and 2mL triethylamine, seal the reaction kettle, stir at room temperature for 30 minutes to obtain a reactant suspension;
[0091] (3) Place the reactant suspension obtained in step (2) in an oven with temperature control function, react at 100°C for 12 hours, centrifuge, then filter, wash with ethanol, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com