Method for synthesizing aromatic polyester without catalyst and product thereof
An aromatic polyester and catalyst technology, applied in the field of polymer material synthesis, can solve the problems of large amount of organic catalysts, low catalytic efficiency, low molecular weight of polyester, etc., and achieve the effect of avoiding biological toxicity and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
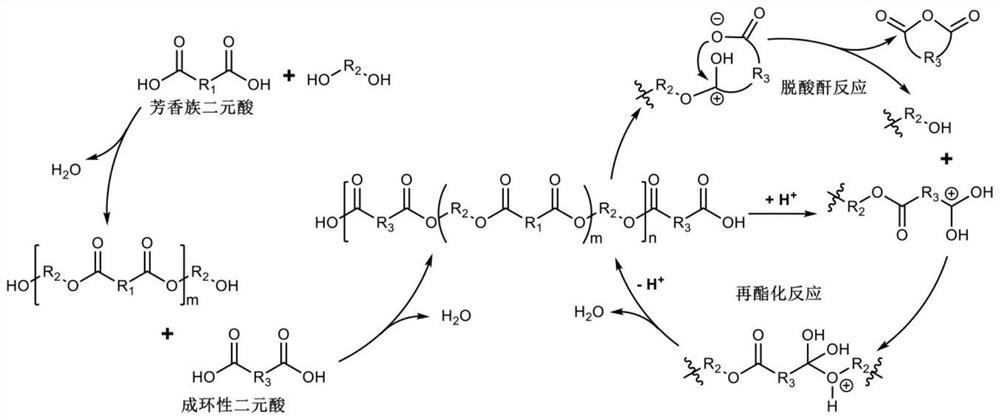
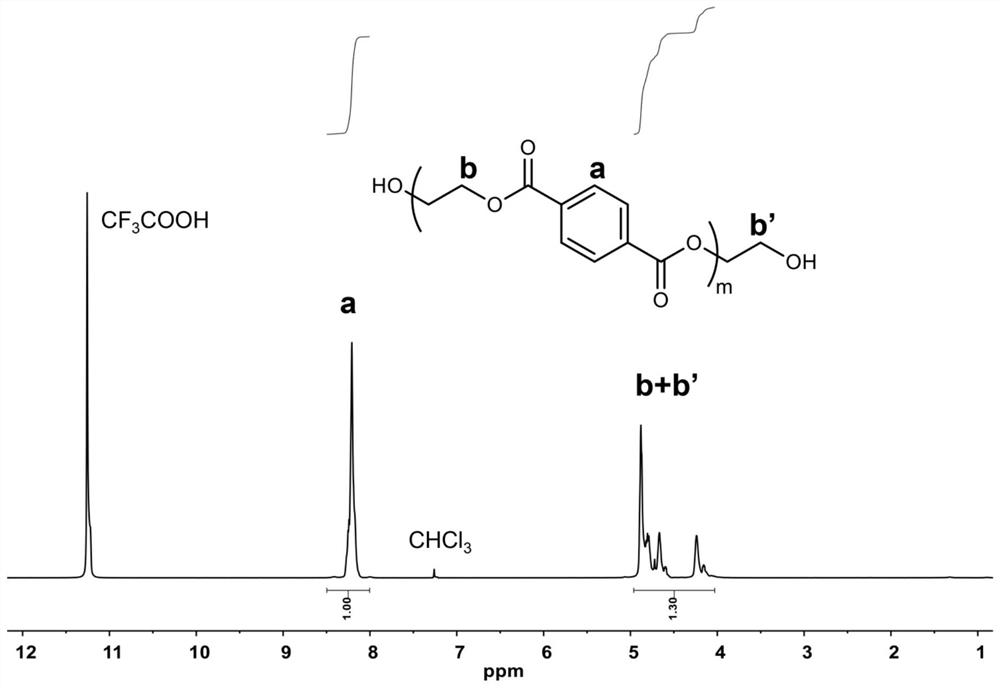
[0028] Prepolymerization stage: Add 52.46g of terephthalic acid (PTA) and 20.00g of ethylene glycol into a 250mL three-necked flask, and heat to 200°C under 0.3MPa pressure to carry out esterification reaction. The reaction time was 2 hours, and a hydroxyl-terminated prepolymer was obtained. through 1 H NMR test shows that in the hydroxyl-terminated prepolymer, the molar ratio of terephthalic acid and ethylene glycol units is 1:1.3.
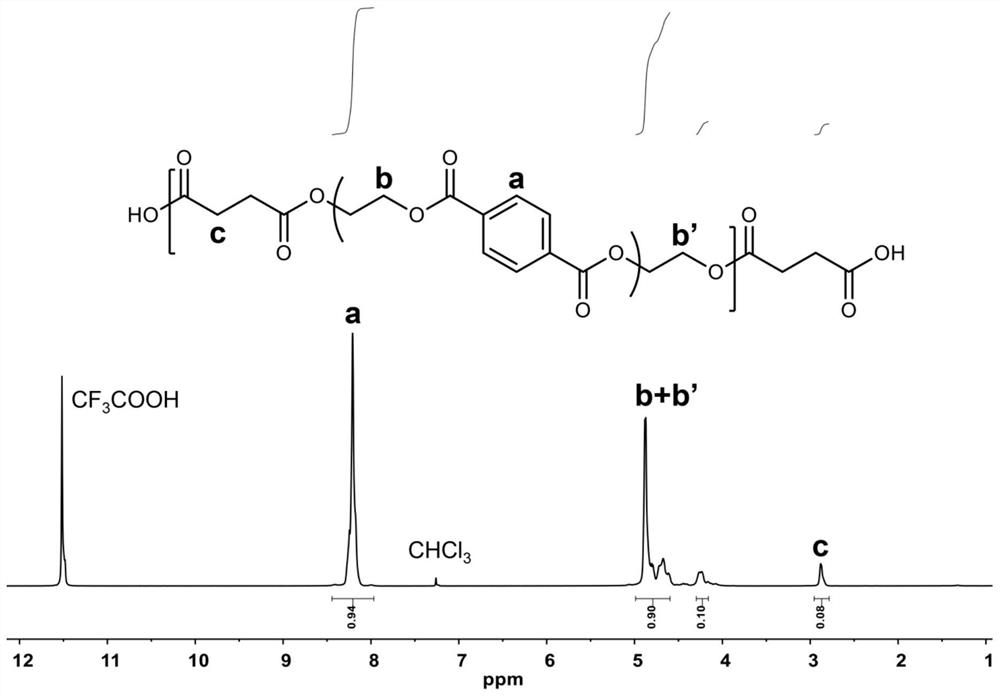
[0029] Then 2.66g of succinic acid (SA) was added, and the molar ratio of terephthalic acid, succinic acid and ethylene glycol was 0.98:0.07:1. The reaction was continued at 200° C. for 2 hours, during which the water generated by the reaction was removed by using a condensation reflux device. After the reaction is completed, the carboxyl-terminated prepolymer is obtained. through 1 H NMR test shows that in the carboxyl-terminated prepolymer, the molar ratio of terephthalic acid, succinic acid and ethylene glycol units is 0.94:0.08:1.
[003...
Embodiment 2~4
[0035] The synthesis process is the same as in Example 1, except that the molar ratios of terephthalic acid, succinic acid and ethylene glycol are replaced by 0.3:0.8:1, 0.6:0.5:1 and 0.95:0.15:1 in sequence.
[0036] After testing, the intrinsic viscosity of the polyester product obtained in Example 2 is 0.78dL / g, and the viscosity average molecular weight is 35500Da.
Embodiment 3
[0037] The intrinsic viscosity of the polyester product obtained in Example 3 is 0.71dL / g, and the viscosity average molecular weight is 31200Da.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com