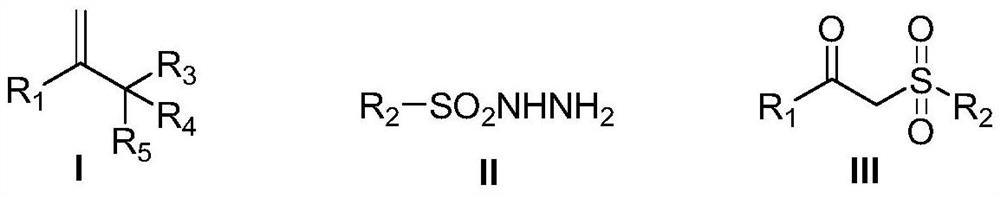
Preparation method of beta-carbonyl sulfone compound
A technology of compound and carbonyl sulfone, which is applied in the field of preparation of β-carbonyl sulfone compounds, can solve the problems of narrow substrate range, large structure restriction of compound A, cumbersome operation, etc., and achieve product purification, easy product and stable process conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
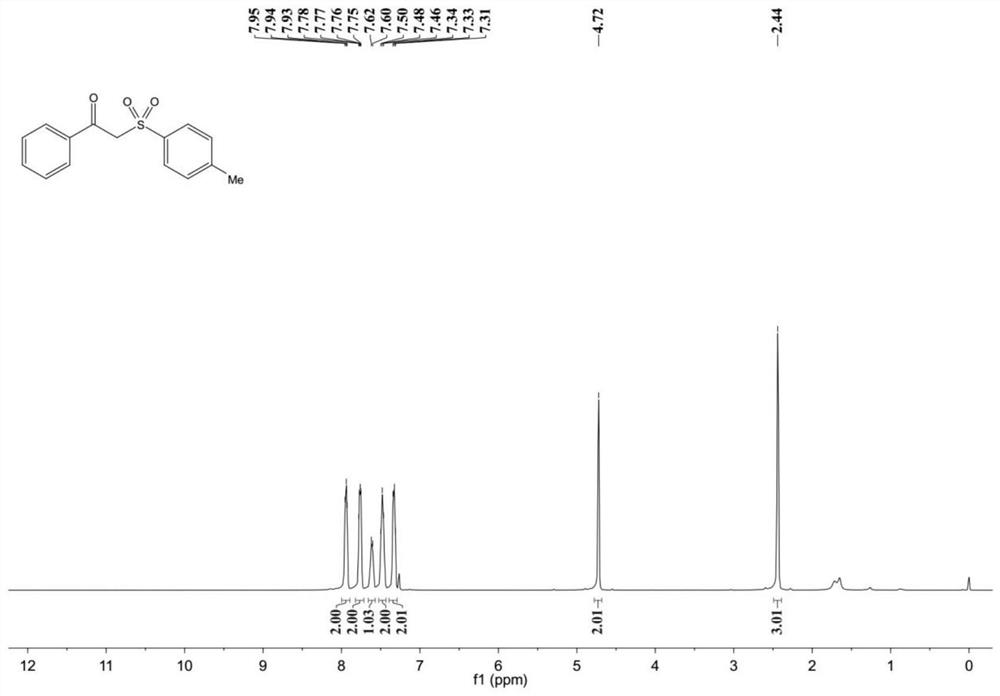
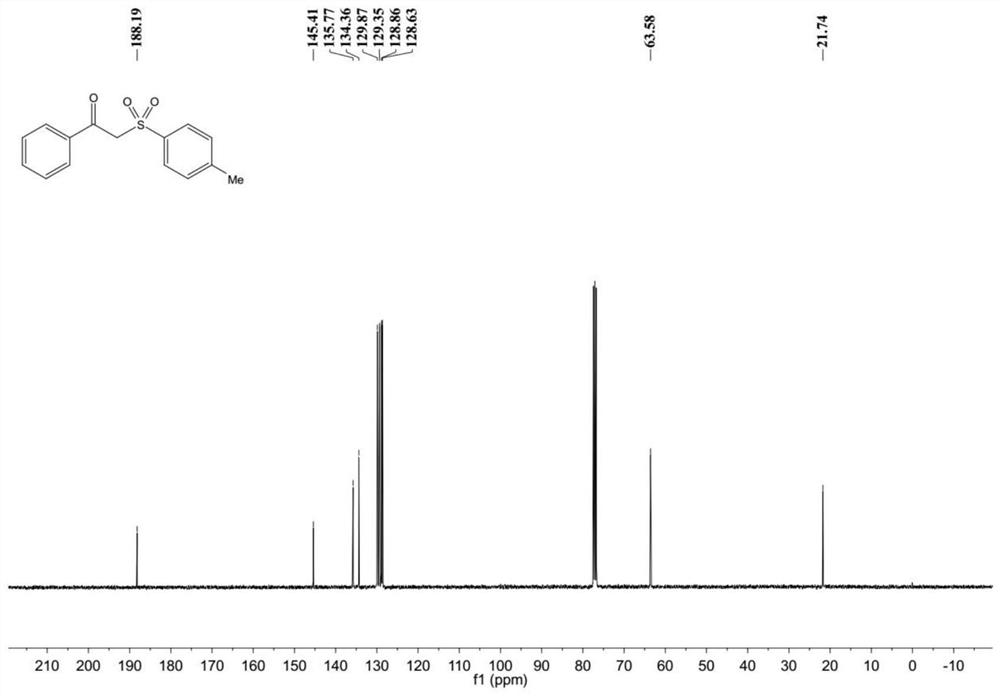
[0039] Example 1: 2-p-toluenesulfonylacetophenone
[0040]
[0041] 2,3-diphenyl-1-propene (97 mg, 0.5 mmol), p-toluenesulfonyl hydrazide (186 mg, 1.0 mmol), copper triflate (9 mg, 0.025 mmol) and acetonitrile / water (V : V=9:1, 2mL) into the reaction tube (25mL), the reaction tube was quickly evacuated, covered with an oxygen balloon, and reacted at 65°C for 12 hours. After the reaction, the solvent was removed under reduced pressure, and the product (white solid, yield 93%) was separated by column chromatography (100-200 mesh, petroleum ether: ethyl acetate = 8:1).
[0042] Product characterization: 1 H NMR (400MHz, CDCl 3 )δ8.00–7.89(m,2H),7.82–7.71(m,2H),7.68–7.57(m,1H),7.53–7.43(m,2H),7.40–7.29(m,2H),4.72( s,2H),2.44(s,3H); 13 C NMR (101MHz, CDCl 3 ) δ 188.19, 145.41, 135.77, 134.36, 129.87, 129.35, 128.86, 128.63, 63.58, 21.74.
Embodiment 2
[0043] Example 2: 1-(4-methylphenyl)-2-p-toluenesulfonyl ethyl ketone
[0044]
[0045] Use 2-p-tolyl-3-phenyl-1-propene (104mg, 0.5mmol) to replace 2,3-diphenyl-1-propene, and the rest are the same as in Example 1, and the reaction operation is carried out to obtain the product (white Solid, yield 62%).
[0046] Product characterization: 1 H NMR (400MHz, CDCl 3 )δ7.85(d, J=8.2Hz, 2H), 7.76(d, J=8.3Hz, 2H), 7.34(d, J=8.0Hz, 2H), 7.28(d, J=8.5Hz, 2H) ,4.69(s,2H),2.45(s,3H),2.43(s,3H); 13 CNMR (101MHz, CDCl 3 ) δ 187.65, 145.59, 145.31, 135.65, 133.28, 129.80, 129.54, 129.50, 128.58, 63.52, 21.80, 21.73.
Embodiment 3
[0047] Example 3: 1-(biphenyl-4-yl)-2-p-toluenesulfonyl ethyl ketone
[0048]
[0049] Use 2-(biphenyl-4-yl)-3-phenyl-1-propene (135mg, 0.5mmol) to replace 2,3-diphenyl-1-propene, the rest are the same as in Example 1, and carry out the reaction operation , to obtain the product (white solid, yield 83%).
[0050] Product characterization: 1 H NMR (400MHz, CDCl 3 )δ8.03(d, J=8.4Hz, 2H), 7.78(d, J=8.2Hz, 2H), 7.71(d, J=8.4Hz, 2H), 7.63(d, J=7.2Hz, 2H) ,7.49(t,J=7.3Hz,2H),7.45–7.40(m,1H),7.35(d,J=8.0Hz,2H),4.75(s,2H),2.45(s,3H); 13 C NMR (101MHz, CDCl 3 )δ 187.68, 147.05, 145.45, 139.44, 135.65, 134.43, 130.04, 129.89, 129.06, 128.64, 128.62, 127.47, 127.35, 63.70, 21.78.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com