A hypoxic fluorescent imaging probe and its preparation method and application
A fluorescent imaging and fluorescent probe technology, applied in the field of chemical imaging, can solve problems such as complex structures and achieve the effect of simple structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
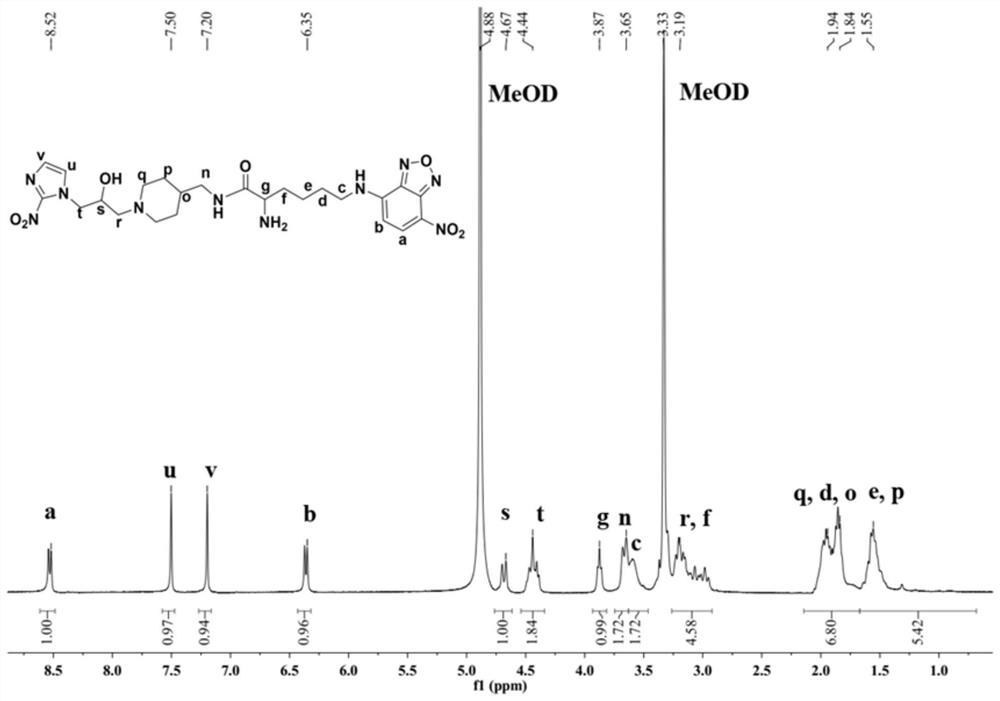
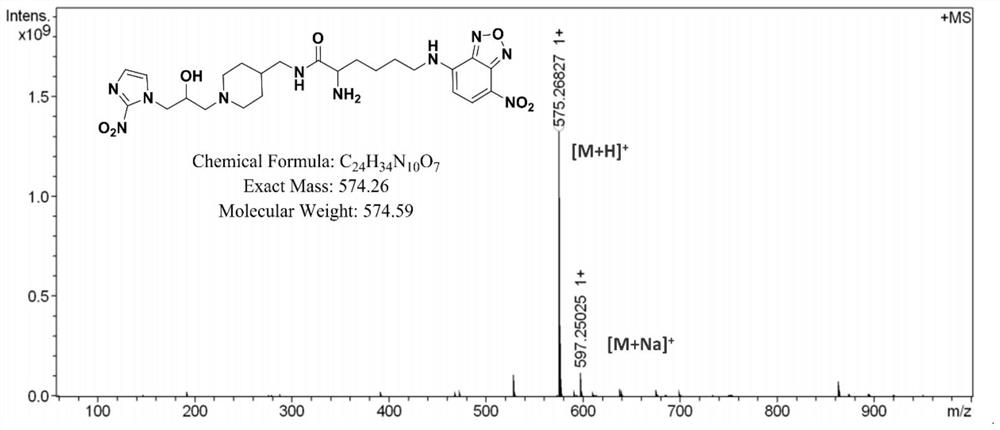
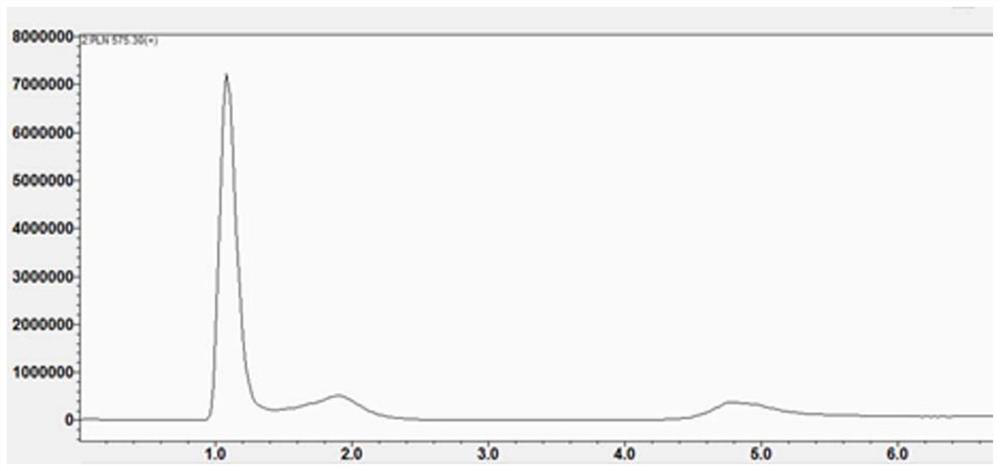
[0069] In this example, the hypoxia fluorescent imaging probe PLN was prepared by the following method:
[0070] (1) Add fmoc-lysine (fmoc-lys, 184mg, 0.5mmol) and sodium carbonate (139mg, 1mmol) into a 50mL round bottom flask, add methanol 6.28mL, water 5mL, stir for about 15min, the solution became clear and fmoc-lys was completely dissolved. 4-Chloro-7nitrobenzo-2-oxa-1,3-oxadiazole (NBD, 100 mg, 0.5 mmol) was dissolved in 2 mL of methanol and added to the reaction system. Stir the reaction at room temperature for 12 hours, adjust the pH value of the reaction solution to 2 with 1M hydrochloric acid, sonicate the reaction solution to fully precipitate, filter and wash the precipitate, dissolve it with methanol and water (10 / 1, V / V), pass through 0.22 μm After filtering the membrane, use high-performance liquid phase, with 20:80 (V / V) as the initial gradient and 100:0 (V / V) as the final gradient to carry out gradient elution, and the gradient elution time is 30min as the sep...
Embodiment 2
[0075] In this example, the hypoxia fluorescent imaging probe PLN was prepared by the following method:
[0076] (1) Add fmoc-lysine (fmoc-lys, 184mg, 0.5mmol) and sodium carbonate (139mg, 1mmol) into a 50mL round bottom flask, add methanol 6.28mL, water 5mL, stir for about 15min, the solution became clear and fmoc-lys was completely dissolved. 4-Chloro-7nitrobenzo-2-oxa-1,3-oxadiazole (NBD, 100 mg, 0.5 mmol) was dissolved in 2 mL of methanol and added to the reaction system. Stir the reaction at room temperature for 12 hours, adjust the pH value of the reaction solution to 2-3 with 1M hydrochloric acid, sonicate the reaction solution to fully precipitate, filter and wash the precipitate, dissolve it with methanol and water (10 / 1, V / V), pass After the 0.22μm filter membrane, use high performance liquid phase, with 20:80 (V / V) as the initial gradient, 100:0 (V / V) as the end gradient, gradient elution is carried out, and the gradient elution time is 30min as the separation cond...
Embodiment 3
[0080] In this example, the hypoxia fluorescent imaging probe PLN was prepared by the following method:
[0081] (1) Add fmoc-lysine (fmoc-lys, 184mg, 0.5mmol) and sodium carbonate (139mg, 1mmol) into a 50mL round bottom flask, add methanol 6.28mL, water 5mL, stir for about 15min, the solution became clear and fmoc-lys was completely dissolved. 4-Chloro-7nitrobenzo-2-oxa-1,3-oxadiazole (NBD, 100 mg, 0.5 mmol) was dissolved in 2 mL of methanol and added to the reaction system. Stir the reaction at room temperature for 12 hours, adjust the pH value of the reaction solution to 2-3 with 1M hydrochloric acid, sonicate the reaction solution to fully precipitate, filter and wash the precipitate, dissolve it with methanol and water (10 / 1, V / V), pass After the 0.22μm filter membrane, use high performance liquid phase, with 20:80 (V / V) as the initial gradient, 100:0 (V / V) as the end gradient, gradient elution is carried out, and the gradient elution time is 30min as the separation cond...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com