Injectable hydrogel dressing with pH response as well as preparation method and application thereof
A technology for injecting water and gel, applied in pharmaceutical formulations, pharmaceutical science, drug delivery, etc., can solve problems such as insufficient hemostasis and healing promotion properties, poor adhesion of hydrogels, and difficulty in application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
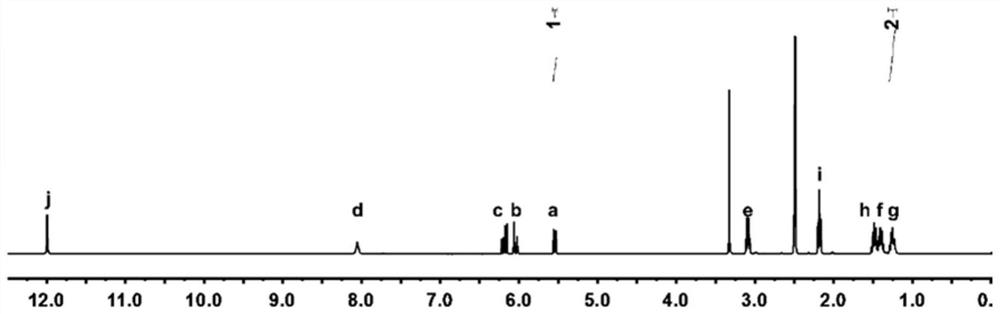
[0059] 1) Preparation of double-bonded amino acid derivatives, acryloyl 6-aminocaproic acid (AA):
[0060] 19.8 g of 6-aminocaproic acid was added to 120 ml of deionized water, and 6.6 g of sodium hydroxide was added to the solution. Stir vigorously in an ice bath until the 6-aminocaproic acid dissolves. 13.35 mL of acryloyl chloride was added to 22.5 mL of tetrahydrofuran solution, and then the acryloyl chloride solution was added dropwise into the aminocaproic acid solution. Adjust and maintain the pH at 7.5-7.8, and react for 24 hours. After the reaction was completed, it was extracted three times with ethyl acetate (100 mL / time). Collect and combine the organic phases. The aqueous phase was acidified to pH 3, and then extracted with ethyl acetate 3 to 5 times (100 mL / time). All the above-mentioned extracted organic phases were collected and combined, dried with sodium sulfate for 18 hours, and then suction filtered using a Buchner funnel. Rotary evaporation is then ca...
Embodiment 2
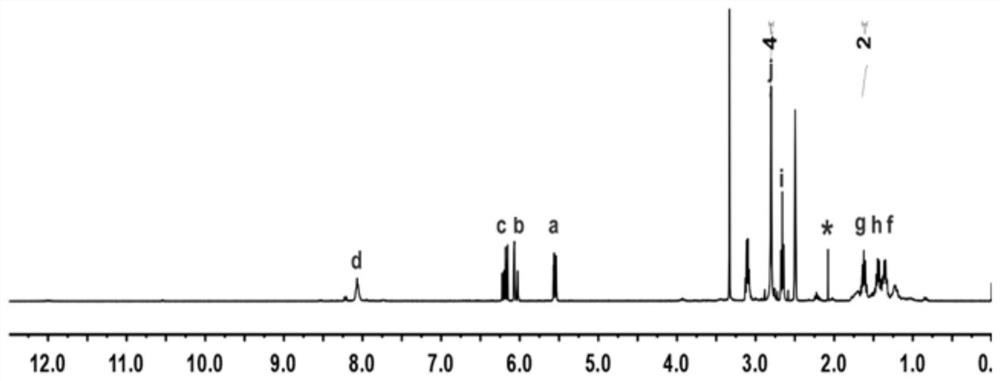
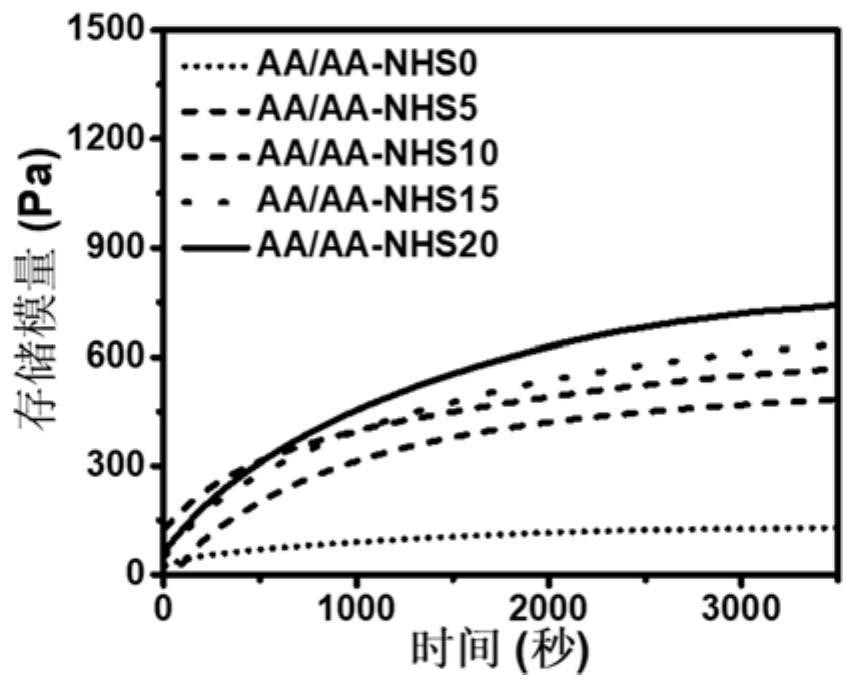
[0066] Dilute the concentration of AA in step 3 to 146 mg / mL, and add 50 μL of 100 mg / mL AA-NHS solution to it, which is prepared with dimethyl sulfoxide. Other conditions were the same as in Example 1 to obtain an AA / AA-NHS5 hydrogel dressing.
Embodiment 3
[0068] Dilute the concentration of AA in step 3 to 138 mg / mL, and add 50 μL of 200 mg / mL AA-NHS solution to it, and other conditions are the same as in Example 2 to obtain AA / AA-NHS10 hydrogel dressing.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adhesive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com