Chain transfer agent with flame retardance and preparation method thereof
A technology of chain transfer agent and flame retardancy, which is applied in the field of chain transfer agent with flame retardancy and its preparation, can solve the problems of easy loss of flame retardant, no flame retardancy, poor dispersibility, etc. Inexpensive, reduced scuffing and wear, and easy-to-use synthetic process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] Add 3-benzylmercaptothiocarbonylpropionic acid (5mmol) into a 100mL branched-necked flask, add 30mL of dichloromethane, slowly add thionyl chloride (7.5mmol) dropwise, react at 40°C for 1.5h, remove low pressure under reduced pressure The boiling point residue was extracted with ethyl acetate / water, the combined organic phases were washed with water, brine, Na 2 SO 4 Dry, filter and concentrate with 10% EtOAc-n-hexane (v / v) as the eluent, and column purification to obtain 3-benzylmercaptothiocarbonylpropionic acid chloride with a yield of 85.4%.
Embodiment 2
[0026]
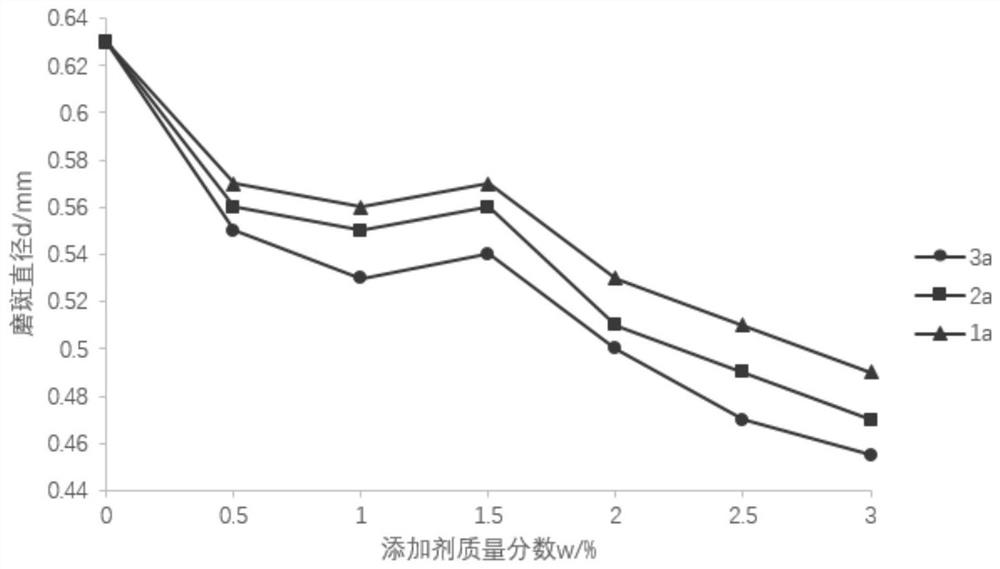
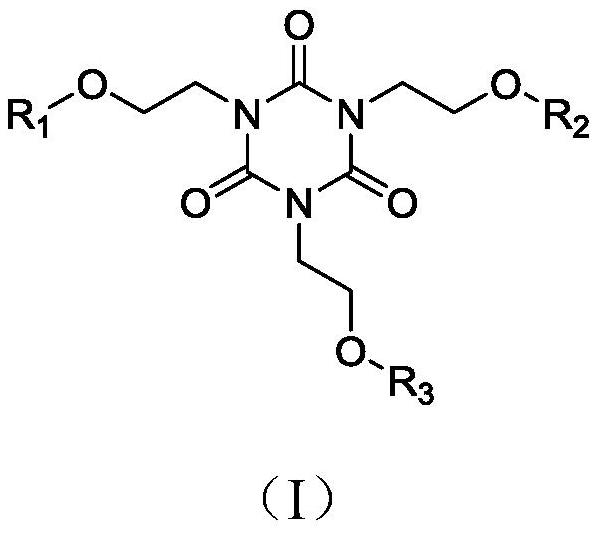
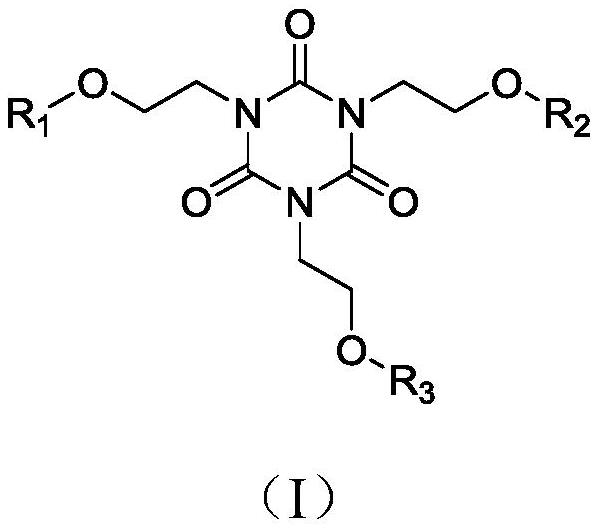
[0027] Add 1,3,5-tris(2-hydroxyethyl)cyanuric acid (5mmol) into a 100mL branched-necked flask, add 20mL of dichloromethane, raise the temperature to 40°C, add pyridine (5mmol) and acid chloride 3-benzene A dichloromethane solution of methylmercaptothiocarbonyl propionic acid (5 mmol) was reacted at 40°C for 3 h. After the reaction was completed, it was extracted with ethyl acetate / water, and the combined organic phases were washed with water and brine, and Na 2 SO 4 After drying, filtering and concentrating, 5% EtOAc-n-hexane (v / v) was used as the eluent, and the column was purified to obtain the flame-retardant chain transfer agent 1a with a yield of 88.1%. 1 H-NMR (400MHz, CDCl 3 ),δ(ppm):7.31-7.25(m,5H,Ph),4.82(s,2H,OH),4.60(s,2H,CH 2 ),4.51(t,2H,CH 2 ),3.63(t,4H,CH 2 ),3.45(t,2H,CH 2 ),3.38(t,4H,CH 2 ),3.27(t,2H,CH 2 ),2.89(t,2H,CH 2 ). 13 C-NMR (100MHz, CDCl 3 ), δ(ppm): 226.31, 171.45, 148.32, 136.15, 128.78, 127.72, 127.21, 60.10, 57.24, 47.83, 45....
Embodiment 3
[0029]
[0030] Add 1,3,5-tris(2-hydroxyethyl)cyanuric acid (5mmol) into a 100mL branched-necked flask, add 20mL of dichloromethane, raise the temperature to 40°C, add pyridine (10mmol) and acid chloride 3-benzene A dichloromethane solution of methylmercaptothiocarbonyl propionic acid (10 mmol) was reacted at 40°C for 3 h. After the reaction was completed, it was extracted with ethyl acetate / water, and the combined organic phases were washed with water and brine, and Na 2 SO 4 After drying, filtering and concentrating, 5% EtOAc-n-hexane (v / v) was used as the eluent, and the column was purified to obtain flame-retardant chain transfer agent 2a with a yield of 82.4%. 1 H-NMR (400MHz, CDCl 3 ),δ(ppm):7.38-7.26(m,10H,Ph),4.91(s,1H,OH),4.67(s,4H,CH 2 ),4.55(t,4H,CH 2 ),3.75(t,2H,CH 2 ),3.53(t,4H,CH 2 ),3.46(t,2H,CH 2 ),3.32(t, 4H,CH 2 ),2.91(t,4H,CH 2 ). 13 C-NMR (100MHz, CDCl 3 ), δ(ppm): 227.24, 172.14, 149.85, 136.96, 129.52, 128.46, 127.87, 61.24, 57.97, 49.20, 45....
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com