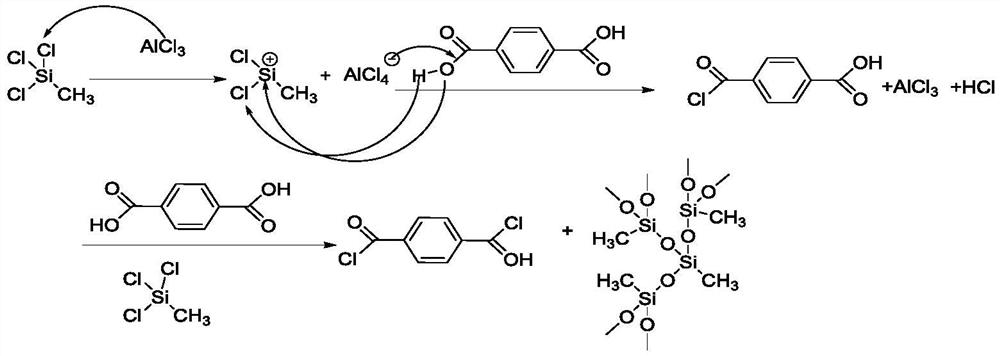
Process for preparing paraphthaloyl chloride
A technology of terephthaloyl chloride and terephthalic acid, applied in the field of chemical raw material preparation, can solve the problems of poor operation safety and protection, unrecyclable by-products, poor product quality controllability and the like, and achieves safe and convenient operation, The effect of high mechanical strength and good paintability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] First, add 149.52g carbon tetrachloride, 49.84g (0.3mol) terephthalic acid, 0.11g (0.0008mol) aluminum trichloride to the three-necked flask, raise the temperature to 40°C, add 59.78g (0.4 mol) methyltrichlorosilane, heat preservation reaction for 6h, hydrogen chloride gas enters the absorption device. After the reaction was completed, the solvent carbon tetrachloride was evaporated under reduced pressure for mechanical use, and then terephthaloyl chloride was evaporated under reduced pressure, cooled, crystallized, and dried to obtain 59.05 g of terephthaloyl chloride with a yield of 96.96%. 99.23% pure. After cooling the mother liquor and adding water to quench the aluminum chloride, the by-product polymethylsilsesquioxane was subjected to solid-liquid separation and dried to obtain 25.94 g of high-quality polymethylsilsesquioxane with a yield of 96.66%.
Embodiment 2
[0035] First, add 199.36g carbon tetrachloride, 49.84g (0.3mol) terephthalic acid, 0.4g (0.00246mol) ferric trichloride to the three-necked flask, raise the temperature to 50°C, and add 61.28g (0.41 mol) methyltrichlorosilane, heat preservation reaction for 8h, hydrogen chloride gas enters the absorption device. After the reaction was completed, the solvent carbon tetrachloride was evaporated under reduced pressure for mechanical use, and then terephthaloyl chloride was evaporated under reduced pressure, cooled, crystallized, and dried to obtain 59.61 g of terephthaloyl chloride with a yield of 97.88%. 99.32% pure. After cooling the mother liquor and adding water to quench ferric chloride, the by-product polymethylsilsesquioxane was subjected to solid-liquid separation and dried to obtain 26.07 g of high-quality polymethylsilsesquioxane with a yield of 97.12%.
Embodiment 3
[0037] First, add 299.04g carbon tetrachloride, 49.84g (0.3mol) terephthalic acid, 0.67g (0.00258mol) tin tetrachloride to the three-necked flask, raise the temperature to 60°C, add 64.27g (0.43 mol) methyltrichlorosilane, heat preservation reaction for 6h, hydrogen chloride gas enters the absorption device. After the reaction was completed, the solvent carbon tetrachloride was evaporated under reduced pressure for mechanical use, and then terephthaloyl chloride was evaporated under reduced pressure, cooled, crystallized, and dried to obtain 60.07 g of terephthaloyl chloride with a yield of 98.63%. 99.21% purity. After cooling the mother liquor and adding water to quench tin tetrachloride, the by-product polymethylsilsesquioxane was subjected to solid-liquid separation and dried to obtain 226.39 g of high-quality polymethylsilsesquioxane with a yield of 98.33%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com