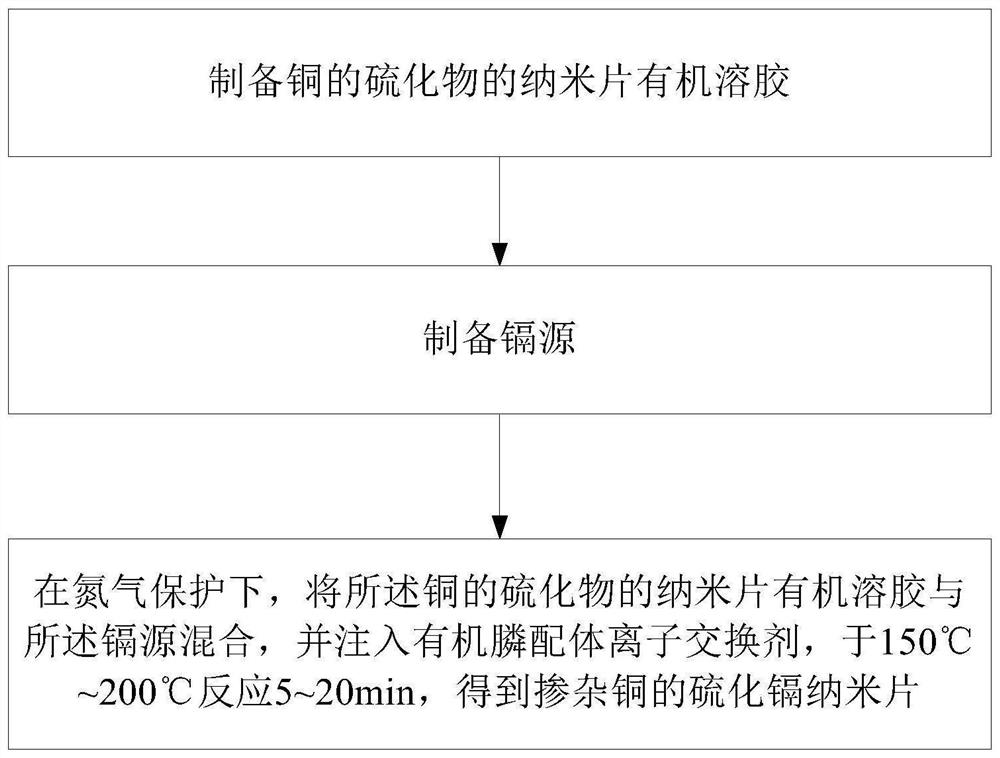
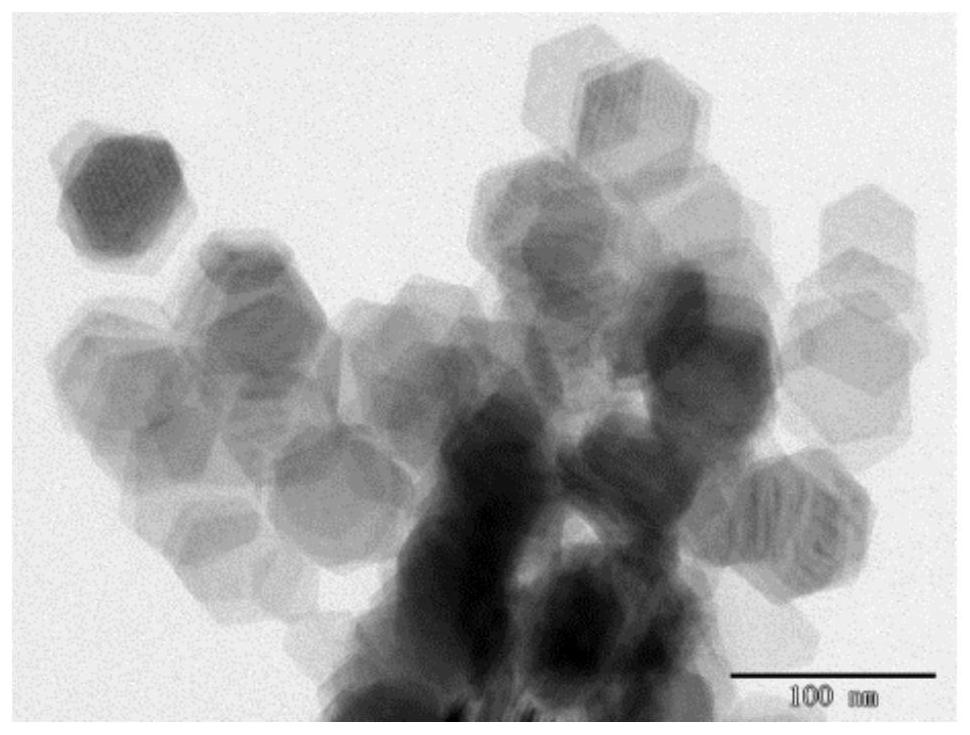
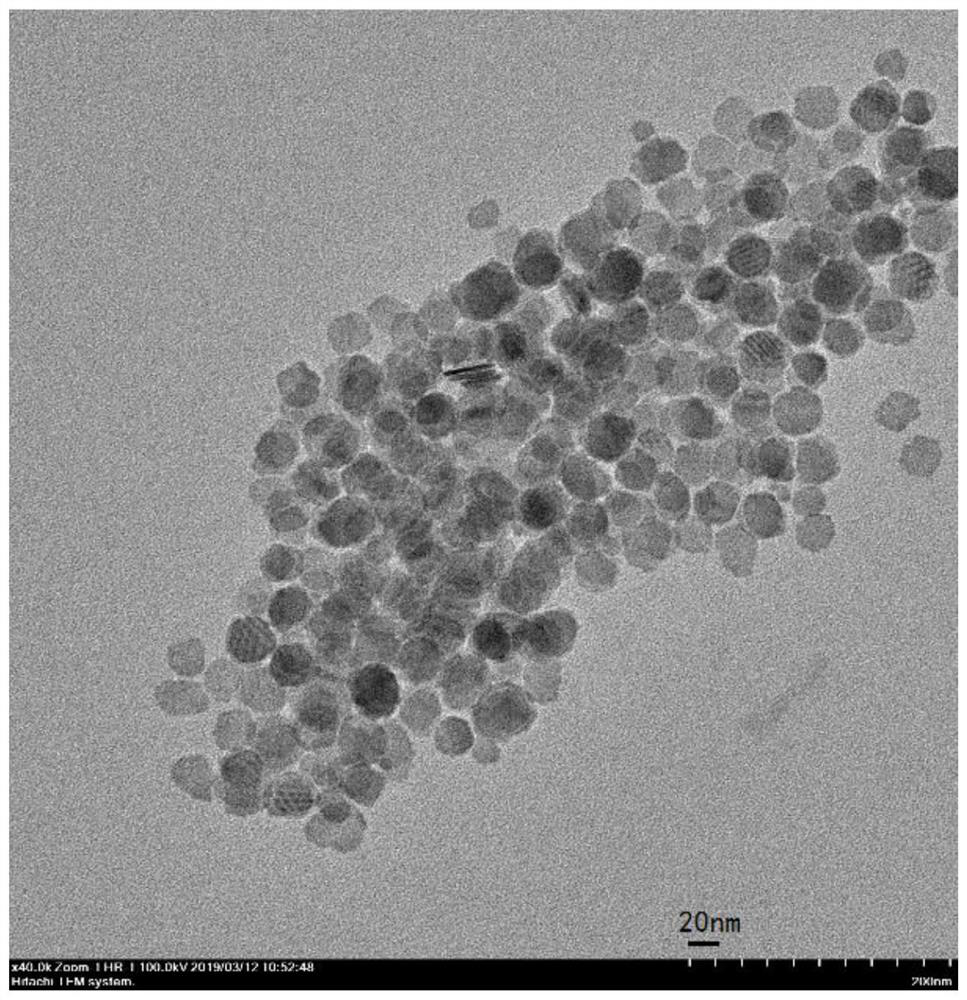
Method for preparing copper-doped cadmium sulfide nanosheets based on ion exchange reaction
An ion-exchange reaction and ion-exchanger technology, which is applied in cadmium sulfide, chemical instruments and methods, nanotechnology, etc., can solve the problems of affecting the stability of the performance of the matrix material, difficult to locate impurities, and unable to obtain cadmium sulfide.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of substituted copper-doped hexagonal cadmium sulfide nanosheets
[0050] Prepare divalent cadmium ion solution:
[0051] 0.031g Cd(NO 3 ) 2 4H 2 O was dissolved in 1 mL of methanol to form a 0.1 mol / L methanol solution of cadmium ions for use.
[0052] Preparation of Cu 2 S nanosheet organosol:
[0053] Take 1 mL of the CuS nanosheet toluene sol prepared above and put it into a single-necked flask, then add 10 mL of octadecene (organic solvent), 0.2 mL of n-dodecyl mercaptan (weak reducing agent), and react at 160 ° C for 30 min under the protection of nitrogen to obtain the obtained Cu reduced to an intermediate valence state 2 S nanosheet organosol.
[0054] Preparation of substituted copper-doped hexagonal cadmium sulfide nanosheets:
[0055] Add the above-mentioned cadmium ion methanol solution to the above-mentioned containing Cu 2 Add 0.2 mL of tributylphosphine (ion exchanger) to the single-necked flask of the S nanosheet organosol, react at...
Embodiment 2
[0059] Preparation of substituted copper-doped cadmium sulfide nanosheets
[0060] Prepare divalent cadmium ion solution:
[0061] 0.031g Cd(NO 3 ) 2 4H 2 O was dissolved in 1 mL of methanol to form a 0.1 mol / L ethanol solution of cadmium ions for later use.
[0062] Preparation of Cu 2 S nanosheet organosol:
[0063] Take 0.5 mL of the CuS nanosheet toluene sol prepared above and put it into a single-necked flask, then add 5 mL of octadecene (organic solvent), 0.1 mL of n-dodecanethiol (weak reducing agent), and react at 170 ° C for 40 min under nitrogen protection to obtain Cu that is reduced to an intermediate valence state 2 S nanosheet organosol.
[0064] Preparation of substituted copper-doped hexagonal cadmium sulfide nanosheets:
[0065] Add the above-mentioned cadmium ion methanol solution to the above-mentioned containing Cu 2 Add 0.1 mL of tributylphosphine (ion exchanger) to the single-necked flask of S nanosheet organosol, react at 160° C. for 10 min unde...
Embodiment 3
[0068] Preparation of substituted copper-doped cadmium sulfide nanosheets
[0069] Prepare divalent cadmium ion solution:
[0070] 0.031g Cd(NO 3 ) 2 4H 2 O was dissolved in 1 mL of methanol to form a 0.1 mol / L methanol solution of cadmium ions for use.
[0071] Preparation of Cu 2 S nanosheet organosol:
[0072] Take 0.2mL of the CuS nanosheet toluene sol prepared above and put it into a single-necked flask, then add 20mL of octadecene (organic solvent), 0.4mL of n-dodecanethiol (weak reducing agent), and react at 180°C for 50min under the protection of nitrogen to obtain Cu that is reduced to an intermediate valence state 2 S nanosheets;
[0073] Preparation of substituted copper-doped hexagonal cadmium sulfide nanosheets:
[0074] Add the above-mentioned cadmium ion methanol solution to the above-mentioned containing Cu 2 Add 0.4 mL of tributylphosphine (ion exchanger) to the single-necked flask of the S nanosheet organosol, react at 160° C. for 10 min under nitrog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com