Extraction system for separating lithium isotopes
A lithium isotope and extraction technology, applied in the field of lithium isotope separation, can solve the problems of expensive production equipment, difficult abundance, small yield, etc., and achieve the effects of shortening exchange equilibrium time, good synergistic extraction, and increasing abundance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The extraction system for separating lithium isotopes provided in this example and the process for lithium isotope separation are as follows:
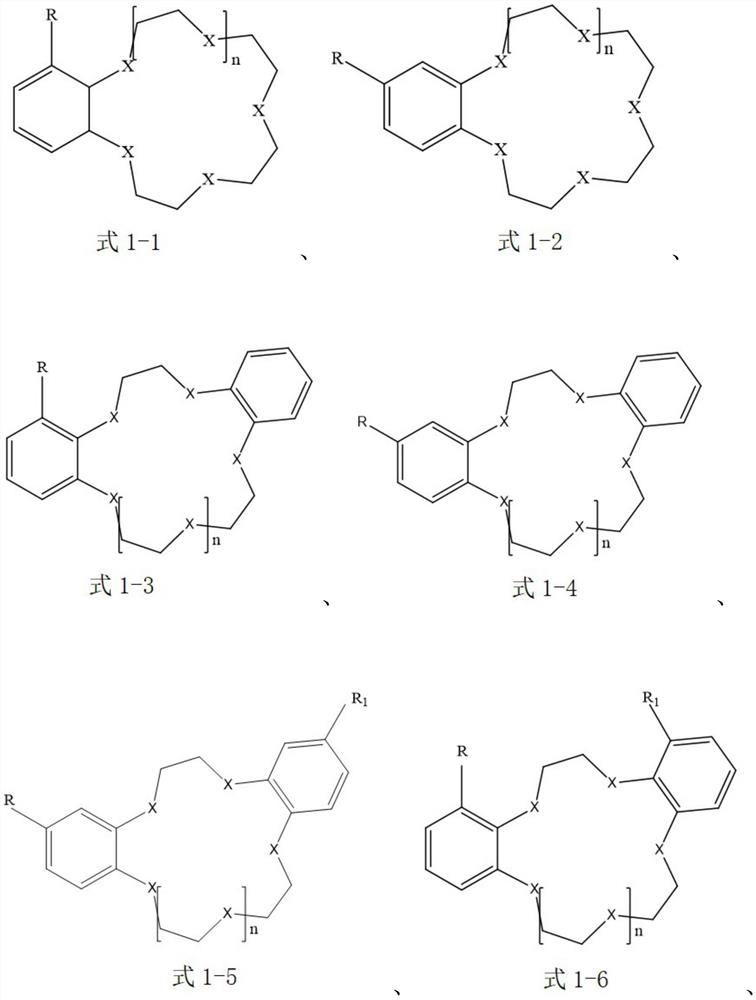
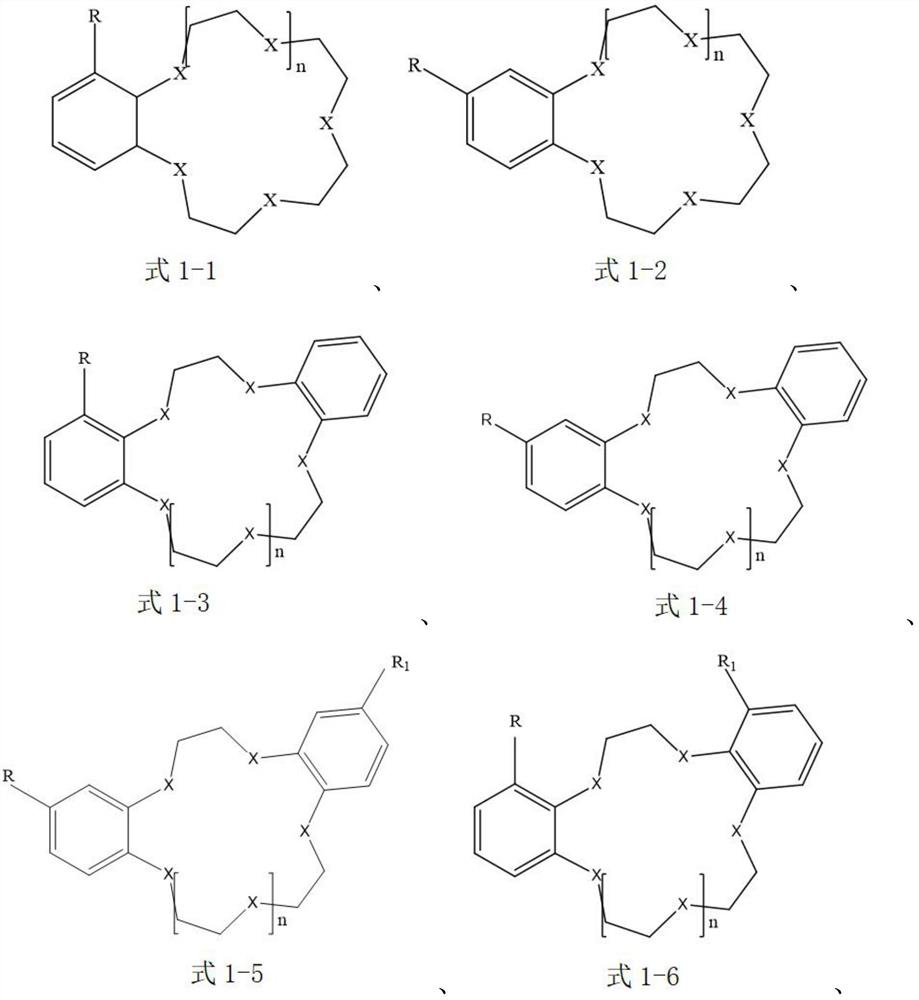
[0044] The extraction agent in the organic extraction phase is selected as benzo-15-crown-5, that is, the compound of the aforementioned formula 1-1; the ionic liquid is selected as 1-butyl-3-methylimidazolium tetrafluoroborophosphate [BMIm][BF 4 ], that is, the cation is selected as the compound of the aforementioned formula 2-9, and the anion is selected as [PF 4 ] - ; The diluent is selected as anisole.
[0045] The lithium salt of the lithium salt solution phase is selected as Li[NTf 2 ].
[0046] The preparation of the organic extraction phase is specifically: the ionic liquid [BMIm] [BF 4 ] and the diluent anisole are mixed with each other according to the volume ratio of 3:7, and then the extractant benzo-15-crown-5 is added to obtain an organic extract phase with an extractant concentration of 0.1mol / L.
[0047] The...
Embodiment 2
[0055] The extraction system for separating lithium isotopes provided in this example and the process for lithium isotope separation are as follows:
[0056] The extraction agent in the organic extraction phase is selected as 4-aminobenzo-15-crown-5, that is, the compound of the aforementioned formula 1-2; the ionic liquid is selected as N-butylpyridine bis(trifluoromethanesulfonyl)imide salt [BPy][NTf 2 ], that is, the cation selection is the compound of the aforementioned formula 2-1, and the anion selection is [(SO 2 CF 3 ) 2 N] - ; The diluent is selected as chloroform.
[0057] The lithium salt of the lithium salt solution phase is selected as CF 3 COOLi.
[0058] The preparation of the organic extraction phase is specifically: the ionic liquid [BPy][NTf 2 ] and the diluent chloroform are mixed with each other according to the volume ratio of 4:6, and then the extractant 4-aminobenzo-15-crown-5 is added to obtain an organic extract with an extractant concentration ...
Embodiment 3
[0067] The extraction system for separating lithium isotopes provided in this example and the process for lithium isotope separation are as follows:
[0068] The extractant in the organic extraction phase is selected as 4-bromobenzo-15-crown-5, that is, the compound of the aforementioned formula 1-2; the ionic liquid is selected as 1-butyl-3-methylimidazole bistrifluoromethanesulfonate imide salt [BMIm][NTf 2 ], that is, the cation selection is the compound of the aforementioned formula 2-9, and the anion selection is [(SO 2 CF 3 ) 2 N] - ; The choice of diluent is 1,1,2-trichloroethane.
[0069] The lithium salt of the lithium salt solution phase is selected as LiI.
[0070] The preparation of the organic extraction phase is specifically: the ionic liquid [BMIm] [NTf 2 ] and the diluent 1,1,2-trichloroethane are mixed with each other according to the volume ratio of 5:5, and then the extractant 4-bromobenzo-15-crown-5 is added to obtain an extractant concentration of 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com