Pharmaceutical composition for treating or preventing heterotopic ossification
A composition and drug technology, applied in the field of anti-ALK2 antibody, can solve problems such as the fundamental treatment method has not yet been established
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0294] Evaluation of BMP Signaling Activation by Anti-ALK2 Antibody (27D-H2L2_LALA) Using a Luciferase Reporter Assay
[0295] The anti-ALK2 antibody (27D-H2L2_LALA) used in the experiment was produced according to the method described in Example 12 of WO2016 / 121908.
[0296]Activation of BMP intracellular signaling mediated by raised anti-ALK2 antibodies was analyzed using a BMP-specific luciferase reporter gene. HEK293A cells were treated with 1×10 4 Cells / well were inoculated into a 96-well white microtiter plate (manufactured by CORNING) for luciferase detection, in DMEM medium containing 10% FBS, at 37°C, 5% CO 2 cultured overnight under conditions. On the next day, using Lipofectamine 2000 (manufactured by Invitrogen), the wild-type and R206H mutant expression plasmids of human or mouse ALK2 were respectively introduced together with pGL4.26 / Id1WT4F-luc (Genes Cells, 7, 949 (2002)). After 3 hours, the medium was replaced with fresh OPTI-MEM I (manufactured by Life Tec...
Embodiment 2
[0299] Fab (27D-H2L2_Fab) and F(ab') of anti-ALK2 antibody (27D-H2L2_LALA) 2 (27D-H2L2_F(ab') 2 ) preparation
[0300] 2)-1
[0301] Fabization of 27D-H2L2_LALA
[0302] Use papain from Papaya latex (SIGMA-ALDRICH) to restrict digestion of 27D-H2L2_LALA, then use HiLoad 26 / 600Superdex 200pg (GE Healthcare) to remove Fc fragments, etc., and then use HiTrap MabSelect SuRe, 1mL ( GE Healthcare) separated unreacted 27D-H2L2_LALA, and recovered Fab.
[0303] 2)-2
[0304] F(ab') of 27D-H2L2_LALA 2 change
[0305] Use endoproteinase (Endoproteinase) Glu-C (SIGMA-ALDRICH) to cut 27D-H2L2_LALA, then use HiTrap MabSelect SuRe, 10mL (GE Healthcare) to separate unreacted 27D-H2L2_LALA, and then use Bio-Scale CHT Type I ,5mL (BIO-RAD) recovered F(ab') 2 .
Embodiment 3
[0307] Evaluation of Fab(27D-H2L2_Fab) and F(ab') of Anti-ALK2 Antibody Using Luciferase Reporter Assay 2 (27D-H2L2_F(ab') 2 Activation of BMP signaling in )
[0308] 27D-H2L2_Fab and 27D-H2L2_F(ab') produced in Example 2 were analyzed using a BMP-specific luciferase reporter gene 2 Activation of BMP-mediated intracellular signal transduction. As a comparison control, 27D-H2L2_LALA, which is a full-length anti-ALK2 antibody, was used. The assay of the luciferase reporter gene was carried out in the same manner as in Example 1.
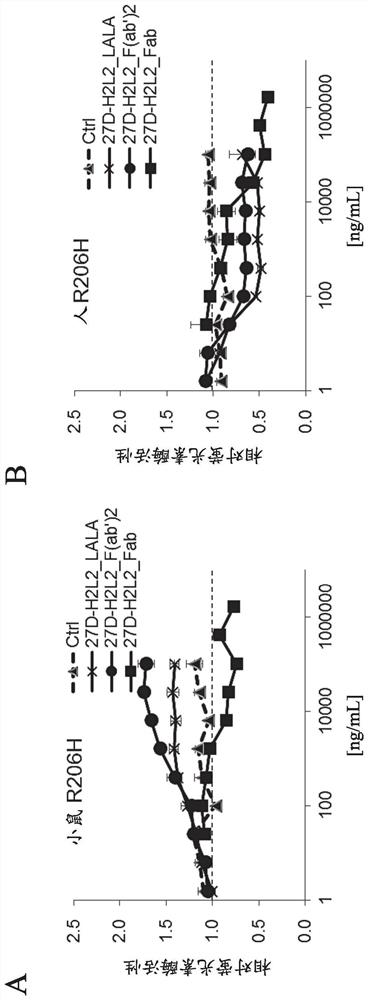
[0309] The result is as figure 2 mentioned. Similar to 27D-H2L2_LALA, 27D-H2L2_F(ab') was confirmed only in HEK293 cells expressing mouse R206H mutant ALK2 2 Increases BMP-specific luciferase activity in a concentration-dependent manner. On the other hand, in 27D-H2L2_Fab, no increase in the activity of the BMP reporter gene was found even under any of the conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com