Fluorescence emission material and organic light-emitting device prepared from fluorescence emission material
A fluorescent emission and furan technology, applied in the direction of luminescent materials, electric solid devices, electrical components, etc., can solve the problems of limited blue fluorescent materials and restricting the development of industrial applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
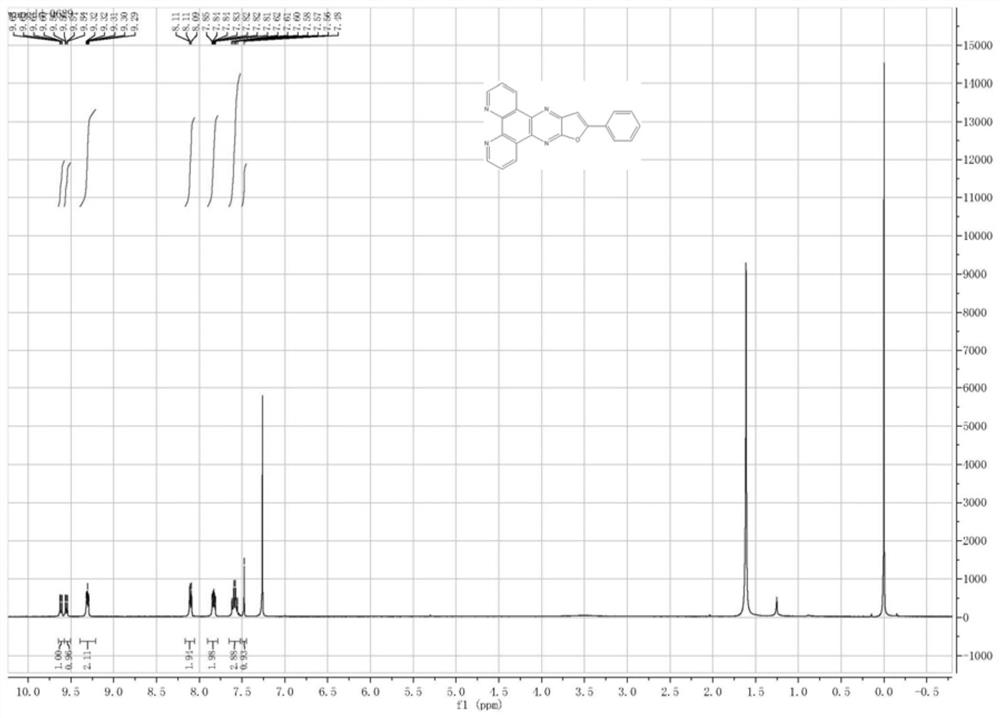
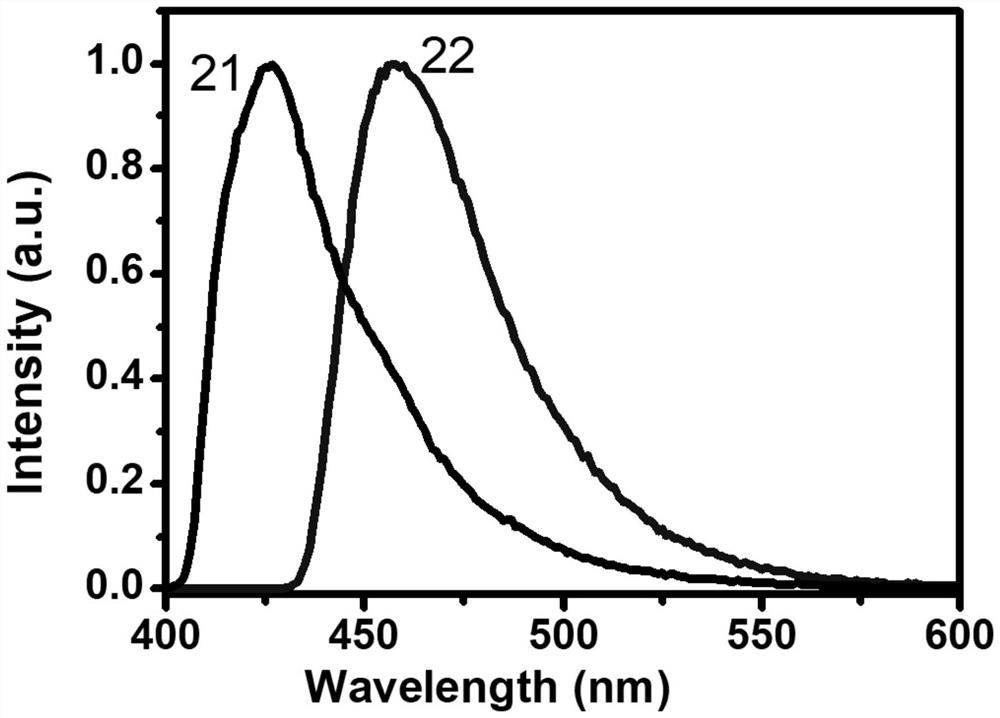
[0061] Synthesis of compound (21)
[0062]
[0063] Synthesis of 5-phenyl-2,3-furandione (M2)
[0064] Dissolve 12g of acetophenone in 150ml of THF, then slowly add 105 mmol of lithium diisopropylamide dropwise at 0°C, stir at 0°C for 30 minutes after the dropwise addition, and then slowly add 15ml of trimethylchlorosilane dropwise, React overnight at room temperature. THF was evaporated after the end of the thin-layer chromatography tracking reaction, then anhydrous hexane was added, filtered, and the filtrate was directly used for the next reaction after further solvent removal.
[0065] The above-prepared intermediate was dissolved in 40 ml of anhydrous ether, and 8.8 ml of oxalyl chloride was slowly added dropwise at room temperature, followed by stirring overnight. After the TLC plate detection reaction was completed, the solid crude product was obtained by direct filtration. The crude product was dissolved in dichloromethane and recrystallized to obtain 6.2 g of M2...
Embodiment II
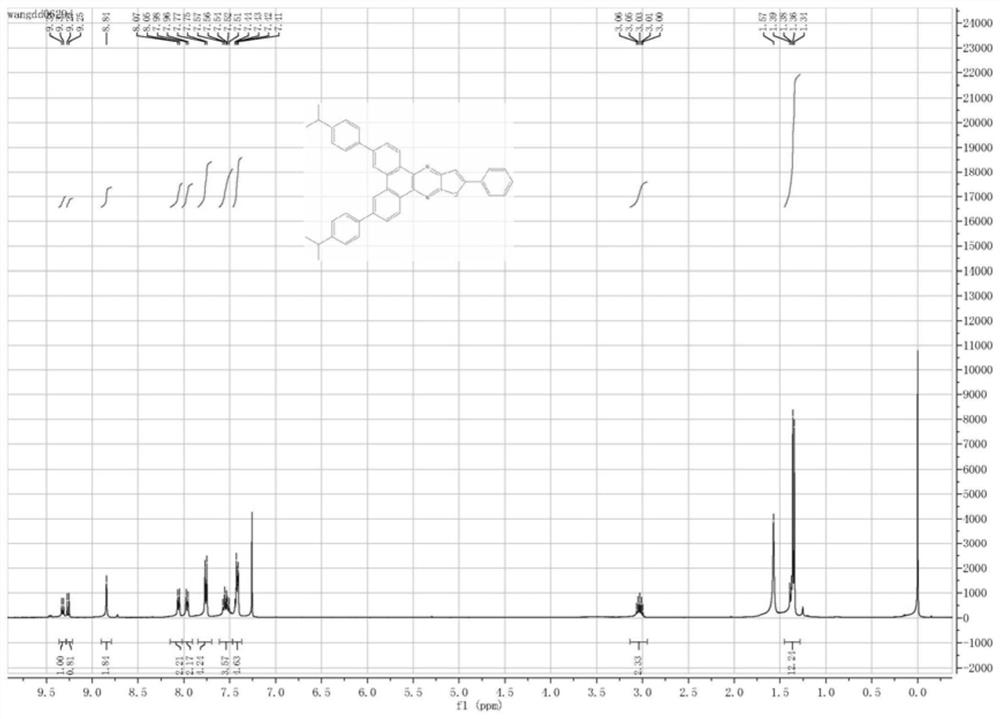
[0071] Embodiment II: the chemical synthesis of compound (22):
[0072] The following figure shows the compound synthesis scheme of compound (22):
[0073]
[0074] (1) Pd(PPh 3 ) 4 , K 2 CO 3 , 1, 4-dioxane: H 2 O=4:1, refluxed; (2) NH 2 OH.HCl, pyridine, ethanol, refluxed; (3) SnCl 2 , contraratedHCl, EtOH, 70°C; (4) acetic acid, refluxed; (5) PPA, 140°C
[0075] Synthesis of 3,6-bis(4-isopropylphenyl)-phenanthrene-9,10-dione (intermediate M4)
[0076] In a dry three-necked flask, replace the air with nitrogen, add 2.2 g of intermediate 2,7-dibromo-9.10-phenanthrene dione, 2.5 g of 4-isopropylphenylboronic acid, 4.46 g of potassium carbonate, and dioxane: Mixed solvent (150ml) of water (1:4), evacuate air for 15 minutes, replace nitrogen, and finally add 0.278 g of tetrakis(triphenyl)phosphine palladium. Heat to reflux and react for two days. After the reaction is detected by TCL, add 100ml of water to the reaction system, and filter to obtain a solid. The obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com