Novel method for preparing sarpogrelate hydrochloride
A technology of sarcogrelate hydrochloride and sarcogrelate hydrochloride, which is applied in the field of preparation of sarcogrelate hydrochloride, can solve problems such as inconvenient operation, difficult crystallization, and unresolved synthesis problems, and achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
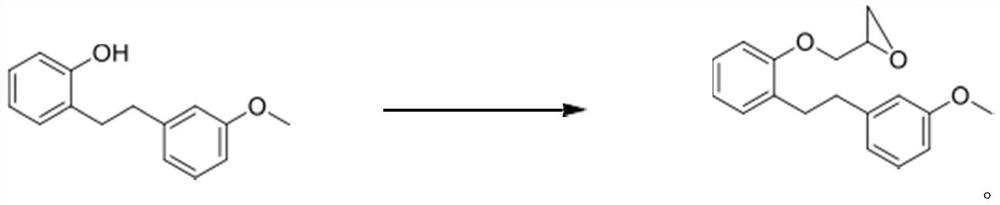
[0034] Step 1 (solvent-free condensation)
[0035]
[0036] Epichlorohydrin (303.9 g) was added to a mixture of 2,3-MPP (150 g) and NaOH (31.5 g) in RB at room temperature and heated at 48 to 52 °C for 3 hours. Another batch of NaOH (5.3 g) was added and heating was continued for 1 h. The reaction was cooled to room temperature and toluene (300ml) was added. The resulting suspension was passed through a bed of water and the filtrate was washed with brine solution (300ml). The volatiles were evaporated in vacuo to give 186 g of a brown oily material.
[0037] analyze:
[0038] MS:284.3(M+H) +
[0039] Step two (ethylene oxide opening)
[0040]
[0041] To a solution of the step 1 intermediate (186 g) in 2-methyl THF (600 ml) was added 40% aqueous dimethylamine (588.7 g) at 5°C. The temperature of the addition was raised to room temperature and stirred for 3h. The reaction mixture was washed with brine solution (300ml). The volatiles were evaporated in vacuo and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com