High performance liquid chromatography method for lisinopramine dimesylate and intermediate impurities thereof
A kind of analytical method, the technology of dextroamphetamine, applied in analytical field, can solve the problems such as no relevant bibliographical reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Chromatographic conditions:
[0058] High performance liquid chromatograph: Dinoex U3000;
[0059] Chromatographic column: Welch Ultimate XB-C8 (150×4.6mm, 5μm);
[0060] Column temperature: 40℃;
[0061] Detection wavelength: 220nm;
[0062] Flow rate: 1.5ml / min;
[0063] Injection volume: 20 μl.
[0064] With 0.2v% triethylamine, 0.02M tetrabutylammonium hydrogen sulfate solution as mobile phase A, phosphoric acid to adjust pH to 7.0, acetonitrile as mobile phase B, gradient elution was performed, and the gradient elution conditions were:
[0065]
[0066]
[0067] Experimental steps:
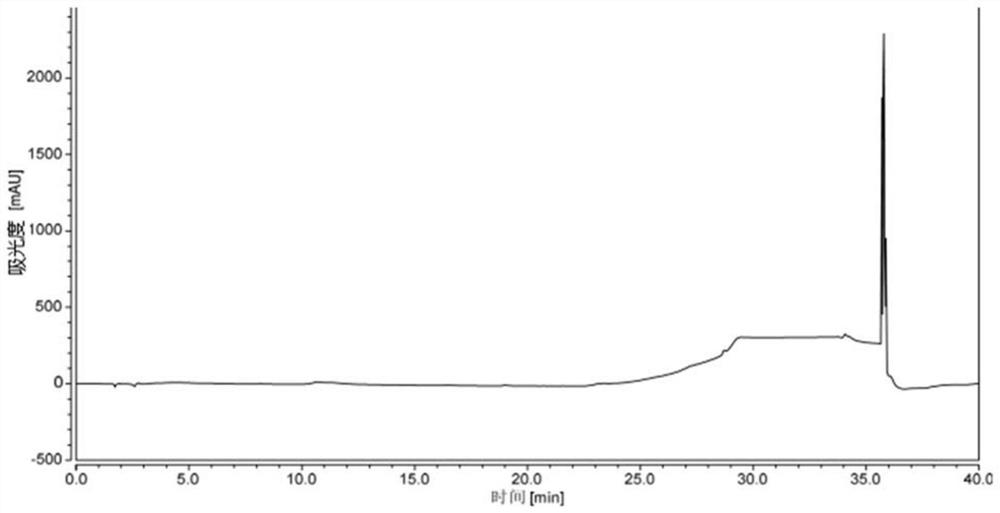
[0068] Take an appropriate amount of lisdexamphetamine dimethanesulfonate and its intermediate impurities, dissolve the sample with methanol respectively, prepare a mixed solution containing about 0.3 mg / ml of lisdexamphetamine dimethanesulfonate and each intermediate, and take an appropriate amount of methanol as a blank solvent . Measure 20 μl of the above solution and i...
Embodiment 2
[0070] High performance liquid chromatograph: Dinoex U3000;
[0071] Chromatographic column: Waters XBridge-C8 (150×4.6mm, 5μm);
[0072] Column temperature: 40℃;
[0073] Detection wavelength: 220nm;
[0074] Flow rate: 1.5ml / min;
[0075] Injection volume: 20 μl.
[0076] Using 0.02M tetrabutylammonium hydrogen sulfate solution containing 0.2v% triethylamine as mobile phase A, adjusting the pH to 7.0 with phosphoric acid, and acetonitrile as mobile phase B, carry out gradient elution. The gradient elution conditions are:
[0077] time (min) Mobile phase A (V%) Mobile phase B (V%) 0 95 5 20 70 30 30 15 85 33 15 85 34 95 5 40 95 5
[0078] Experimental steps:
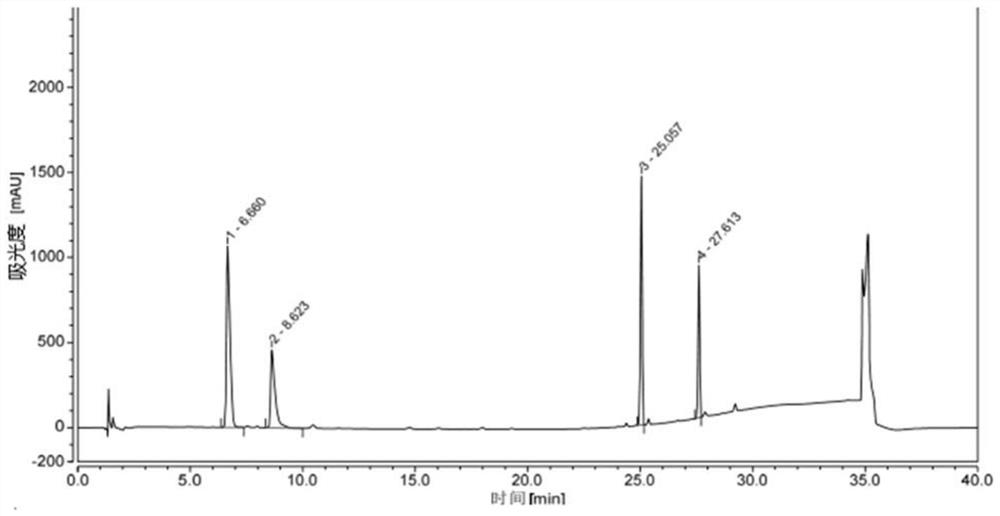
[0079] Get an appropriate amount of lisdexamphetamine dimethanesulfonate and its intermediate impurities, dissolve the sample with methanol respectively, prepare a mixed solution containing about 0.3 mg / ml of lisdexamphetamine dimethanesulfonate and each intermed...
Embodiment 3
[0081] High performance liquid chromatograph: Dinoex U3000;
[0082] Chromatographic column: Welch Ultimate XB-C8 (150×4.6mm, 5μm);
[0083] Column temperature: 40℃;
[0084] Detection wavelength: 220nm;
[0085] Flow rate: 1.5ml / min;
[0086] Injection volume: 20 μl.
[0087] Using 0.2v% triethylamine and 0.01M tetrabutylammonium hydrogen sulfate solution as mobile phase A, pH adjusted to 7.0 with phosphoric acid, and acetonitrile as mobile phase B, gradient elution was performed. The gradient elution conditions were:
[0088] time (min) Mobile phase A (V%) Mobile phase B (V%) 0 95 5 20 70 30 30 15 85 33 15 85 34 95 5 40 95 5
[0089] Experimental steps:
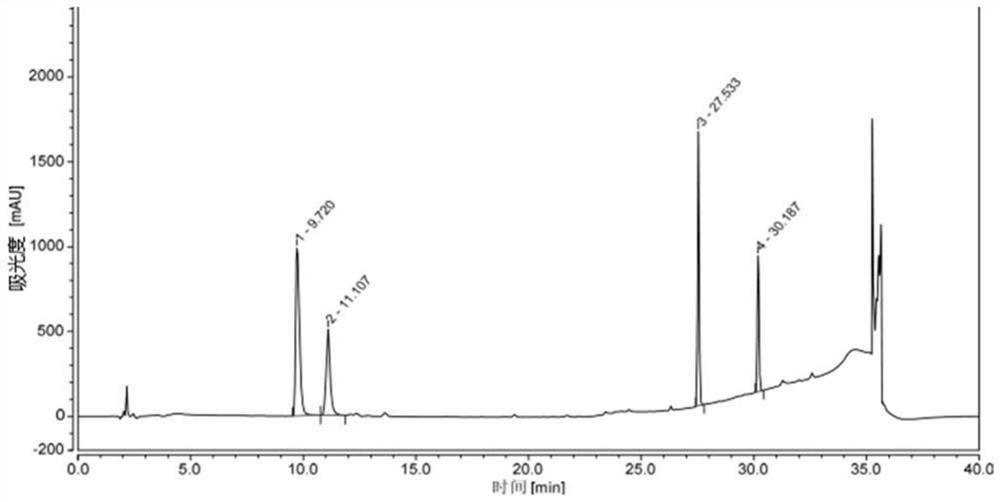
[0090] Get an appropriate amount of lisdexamphetamine dimethanesulfonate and its intermediate impurities, dissolve the sample with methanol respectively, prepare a mixed solution containing about 0.3 mg / ml of lisdexamphetamine dimethanesulfonate and each intermediate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com