A kind of preparation method of ceftizoxime sodium for injection
A technology for ceftizoxime sodium and ceftizoxime acid, which is applied in the field of medicine, can solve the problems of poor product color grade, influence product quality, complicated process, etc., and achieves simple process operation, improved product quality, and powder flow. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
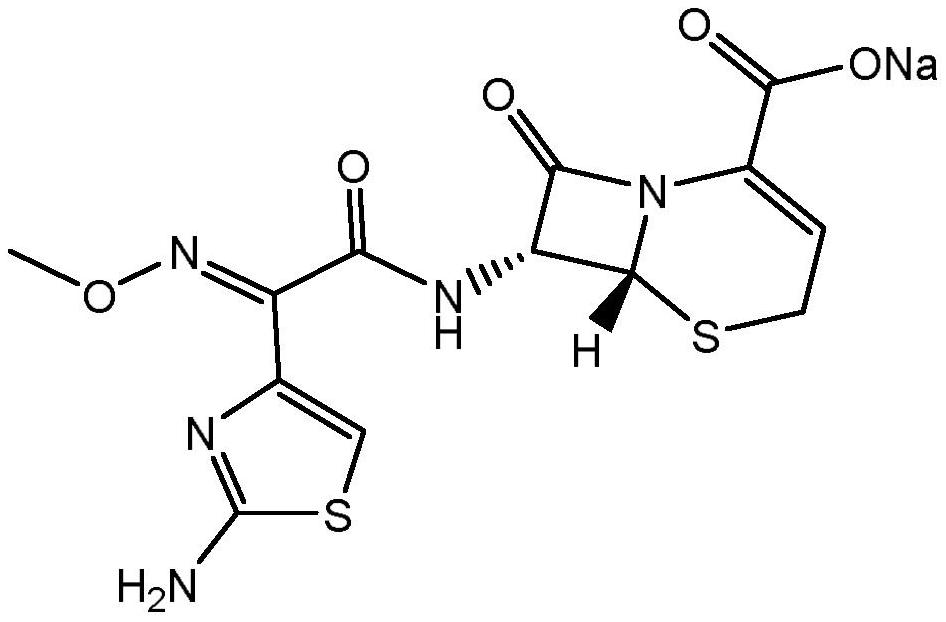
[0033] The invention discloses a method for preparing ceftizoxime sodium for injection. The produced ceftizoxime sodium is easy to pack into powder injection preparations.
[0034] The preparation method comprises the following steps:
[0035] (a) Reduce the temperature of the purified water to 0-5°C; add ceftizoxime acid into the purified water, and add a buffer solution; wherein, the amount of purified water is 2.0-2.5 times the weight of the ceftizoxamic acid. Preferably, the buffer used is acetic acid-sodium acetate buffer; the added amount is 0.1-0.2 times the weight of ceftizoxime acid. The preparation method of acetic acid-sodium acetate buffer solution is as follows: take 54.6g of sodium acetate, add 20mL of 1mol / L acetic acid solution to dissolve, then add water to dilute to 500mL.
[0036] (b) Add a salt-forming agent to the solution obtained in step (a), control the temperature at 0-5° C., and stir until it dissolves. Preferably, the salt-forming agent is sodium a...
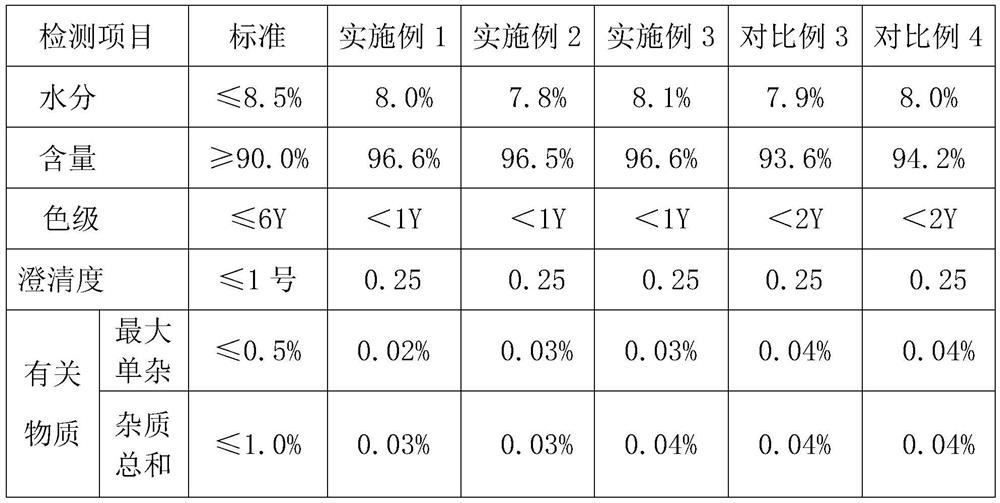
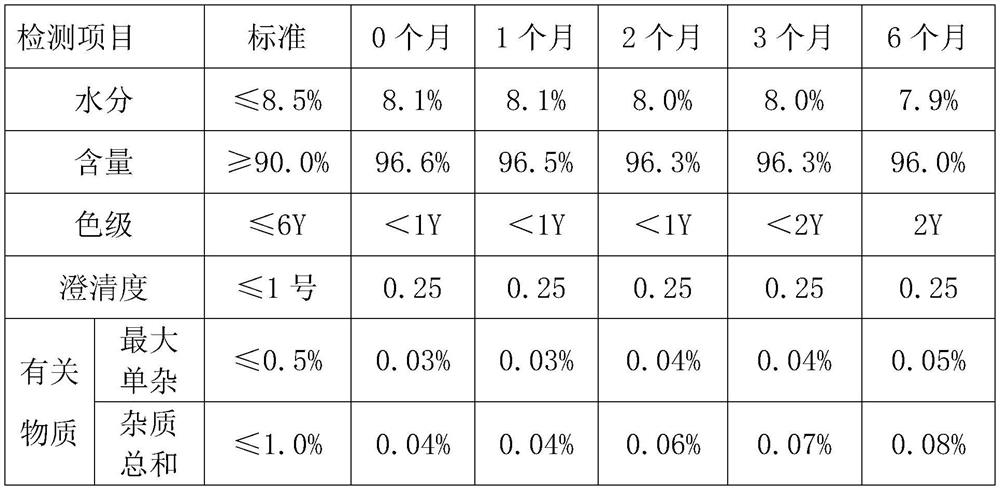
Embodiment 1
[0043] Use the above-mentioned method to prepare ceftizoxime sodium for injection. The specific operation content is as follows:
[0044] Add 60mL of purified water to the reaction tank, cool down to 0-5°C, add 30.0g of ceftizoxime acid under stirring conditions, add 3g of acetic acid-sodium acetate buffer solution under stirring conditions, control the system temperature at 0-2°C, and slowly Add sodium acetate solution (6.75g of sodium acetate dissolved in 120mL of purified water), stir well until it dissolves. After dissolving, add 3.0 g of sodium chloride to the feed liquid, and stir again until dissolving.
[0045] Add 3.0 g of activated carbon to the solution and stir for 60 minutes, then filter, and enter the crystallization tank through sterile filtration, wash with 10 mL of purified water, and merge into the crystallization tank.
[0046] Control the crystallization temperature at 15°C, add 210 mL of ethanol, add 1.5 g of sterile ceftizoxime sodium seed crystals, and...
Embodiment 2
[0048]Add 70mL of purified water to the reaction tank, cool down to 0-5°C, add 30.0g of ceftizoxime acid under stirring conditions, add 6g of acetic acid-sodium acetate buffer solution under stirring conditions, control the temperature of the system at 0-5°C, take 120min to slowly Add sodium acetate solution (7.38g of sodium acetate dissolved in 150mL of purified water), and stir until it dissolves. After dissolving, add 6.0 g of sodium chloride to the feed liquid, and stir again until dissolving.
[0049] Add 4.5 g of activated carbon to the solution and stir for 30 minutes, then filter, enter the crystallization tank through sterile filtration, wash with 10 mL of purified water, and merge into the crystallization tank.
[0050] Control the crystallization temperature at 13°C, add 240 mL of ethanol, add 2.1 g of sterile ceftizoxime sodium seed crystals, grow the crystals for 60 minutes, and add 1800 mL of ethanol after a large amount of crystals have emerged, and control the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com