A chain structure rare earth europium (iii) coordination polymer and its preparation method and application
A technology of coordination polymer and chain structure, applied in the field of fluorescent probes, achieves the effects of simple device, easy separation and purification of products, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 0.08mmol 2,4-bis(4-methylbenzoyl)isophthalic acid and 0.04mmol 2,5-bis(4-methylbenzoyl)terephthalic acid into a round-bottomed flask, Add a mixed solvent composed of 15ml of water and DMF (volume ratio 1:2), heat to reflux to dissolve, then add 0.09mmol of europium nitrate hexahydrate to the mixed solution, stir, continue to heat to reflux, cool, and mix the obtained The solution was transferred to a glass test tube. Add 3ml of a mixed solvent consisting of water and ethanol (volume ratio 1:2) to the liquid surface in the test tube, and then add 4ml of ethanol solution in which 0.23mmol of phenanthroline is dissolved, and the solution in the test tube is layered. The preservative film that has punched small hole is covered test tube mouth, left standstill at room temperature, obtained colorless crystal product in the middle layer of test tube after three weeks.
Embodiment 2
[0038] Add 0.07mmol 2,4-bis(4-methylbenzoyl)isophthalic acid and 0.05mmol 2,5-bis(4-methylbenzoyl)terephthalic acid into a round-bottomed flask, Add 15ml of water and DMF (volume ratio 1:2) mixed solvent, heat to reflux to dissolve, then add 0.08mmol europium nitrate hexahydrate to this mixed solution, stir, continue to heat to reflux, cool, and mix the obtained The solution was transferred to a glass test tube. On the liquid surface in the test tube, first add 3ml of a mixed solvent composed of water and methanol (volume ratio 1:2), and then add 5ml of ethanol solution in which 0.23mmol of phenanthroline is dissolved, and the solution in the test tube is layered. The preservative film that has punched aperture is covered test tube mouth, left standstill at room temperature, obtained the same crystal product as embodiment 1 in the middle layer of test tube after three weeks.
Embodiment 3
[0040] Add 0.09mmol 2,4-bis(4-methylbenzoyl)isophthalic acid and 0.04mmol 2,5-bis(4-methylbenzoyl)terephthalic acid into a round bottom flask, Add 15ml of water and DMF (volume ratio 1:2) mixed solvent, heat to reflux to dissolve, then add 0.08mmol europium nitrate hexahydrate to this mixed solution, stir, continue to heat to reflux, cool, and mix the obtained The solution was transferred to a glass test tube. On the liquid surface in the test tube, first add 3ml of a mixed solvent composed of water and methanol (volume ratio 1:2), and then add 6ml of methanol solution in which 0.23mmol of phenanthroline is dissolved, and the solution in the test tube is layered. The preservative film that has punched aperture is covered test tube mouth, left standstill at room temperature, obtained the same colorless crystal product as embodiment 1 in the middle layer of test tube after three weeks.
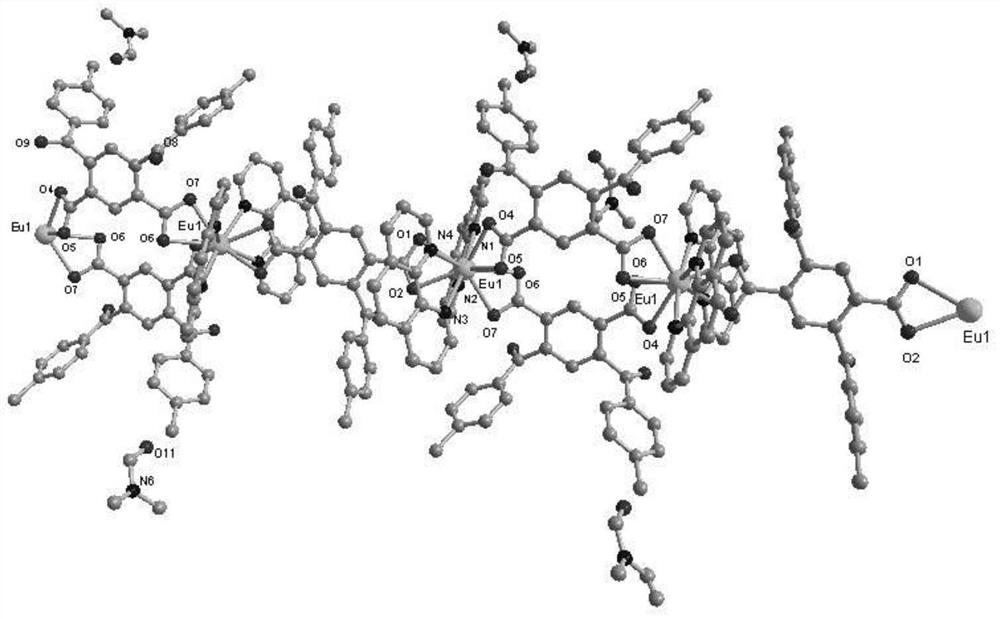
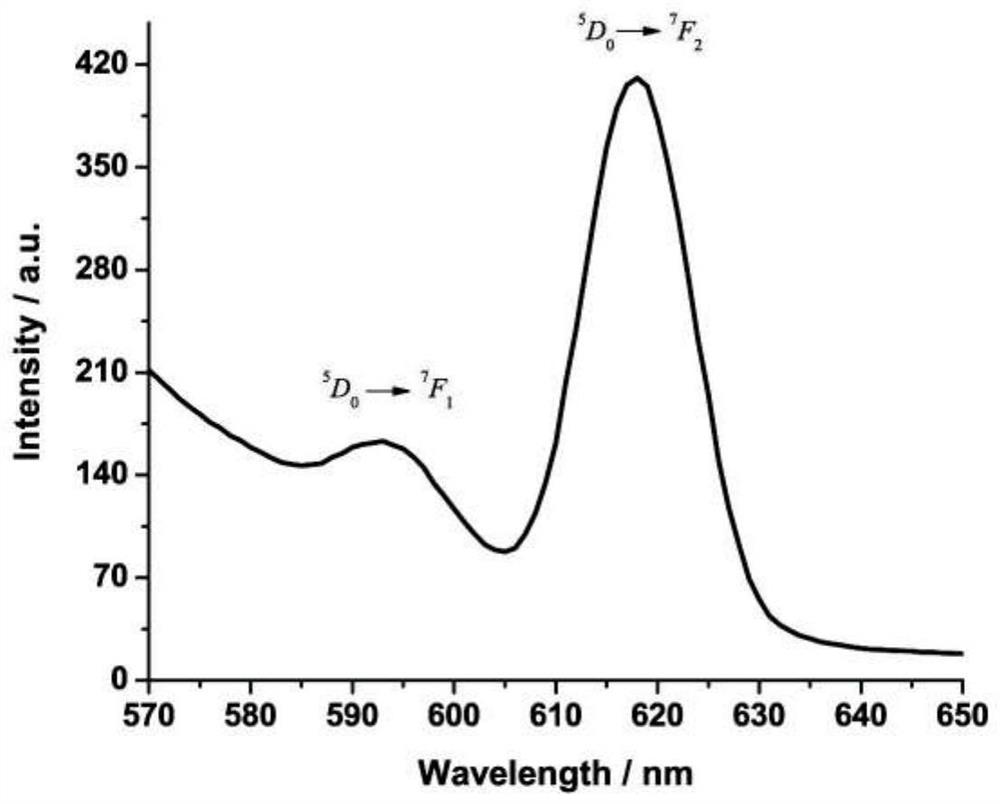
[0041] For the molecular structure of the chain structure rare earth europium (III) coordin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com