Chimeric antigen receptor and application thereof
A technology of chimeric antigen receptors and antigens, applied in the fields of application, antibody medical components, carriers, etc., can solve the problems of ineffective effects, etc., and achieve the goal of releasing immunosuppressive effects, enhancing anti-tumor ability, and good in vivo expansion ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Construction of Chimeric Antigen Receptor
[0050] (1) Synthesize CAR (1928z CAR and T28z CAR) domains through the whole gene, such as figure 1 shown;
[0051] The amino acid sequence of the chimeric antigen receptor is as follows:
[0052] 1928z CAR (SEQ ID NO.2):
[0053] MLLLVTSLLLCELPHPAFLLIPDIQMTQTTSSLSASLGDRVTISCRASQDISKYLNWYQQKPDGTVKLLIYHTSRLHSGVPSRFSGSGSGTDYSLTISNLEQEDIATYFCQQGNTLPYTFGGGTKLEITGSTSGSGKPGSGEGSTKGEVKLQESGPGLVAPSQSLSVTCTVSGVSLPDYGVSWIRQPPRKGLEWLGVIWGSETTYYNSALKSRLTIIKDNSKSQVFLKMNSLQTDDTAIYYCAKHYYYGGSYAMDYWGQGTSVTVSSAAAIEVMYPPPYLDNEKSNGTIIHVKGKHLCPSPLFPGPSKPFWVLVVVGGVLACYSLLVTVAFIIFWVRSKRSRLLHSDYMNMTPRRPGPTRKHYQPYAPPRDFAAYRSRVKFSRSADAPAYQQGQNQLYNELNLGRREEYDVLDKRRGRDPEMGGKPRRKNPQEGLYNELQKDKMAEAYSEIGMKGERRRGKGHDGLYQGLSTATKDTYDALHMQALPPRQAKRKPRKAPSRNICYDAFVSYSERDAYWVENLMVQELENFNPPFKLCLHKRDFIPGKWIIDNIIDSIEKSHKTVFVLSENFVKSEWCKYELDFSHFRLFDENNDAAILILLEPIEKKAIPQRFCKLRKIMNTKTYLEWPMDEAQREGFWVNLRAAIKSTSKIVAPVKQTLNFDLLKLAGDVESNPGPASMVSKGEELFTGVVPILVE...
Embodiment 2
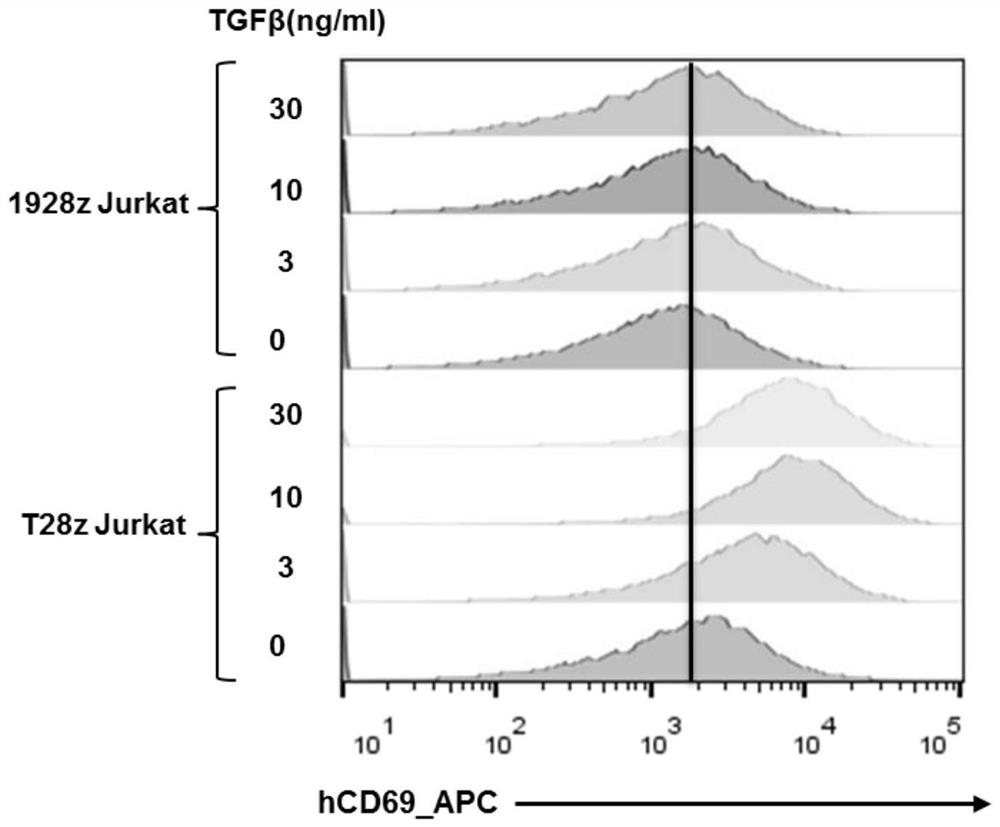
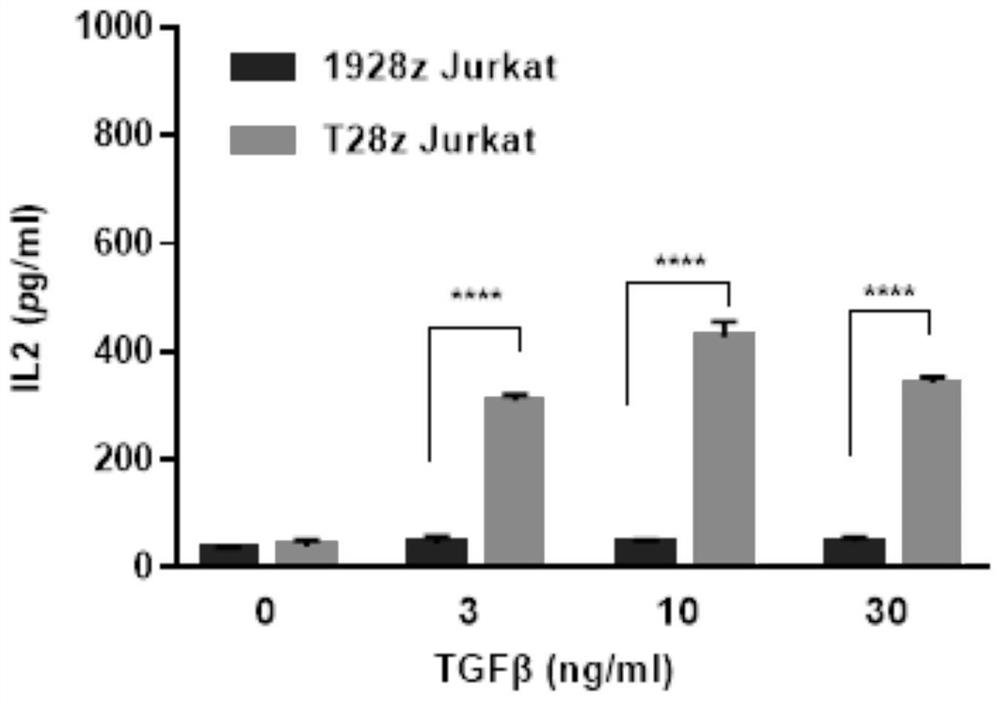
[0056] Example 2: TGFβ activates CAR-jurkat cells in vitro
[0057] The expression plasmids of 1928z CAR-T cells and T28z CAR-T cell vectors in Example 1 were used to prepare CAR-expressing lentiviruses from 293T cells, and finally infected Jurkat cells. The specific steps are as follows:
[0058] (1) put 10 5 CAR Jurkat cells were inoculated into 48-well plates, and TGFβ (0, 3, 10, 30 ng / ml) was added respectively, with a volume of 200 μl, and co-cultured at 37°C for 24 hours;
[0059] (2) After culturing for 24 hours, the 48-well plate was taken out, and the cell supernatant was frozen at -20°C for detection of IL2, and the cells were used for flow cytometry;
[0060] (3) After washing the cells with PBS, add an appropriate amount of APC-labeled human-CD69 antibody, incubate at room temperature for 20 minutes, wash with PBS, and then detect with a flow cytometer;
[0061] (4) Cell supernatant The expression of IL2 in the cell supernatant was detected with an ELISA kit.
...
Embodiment 3
[0063] Example 3: T28z CAR-T immunized mice have no toxic side effects
[0064] In order to verify the safety of T28z CAR-T cells, this example constructed mouse-derived CAR-T cells (since the scFv of TGFβ can be activated by both human-derived and mouse-derived TGFβ, the mouse-derived version of T28z CAR-T The scFv can be used universally), using m1928z T cells that can target mouse B cells as a positive control, and evaluating the safety of T28zT cells by observing the physiological state of mice, as follows:
[0065] (1) Prepare retrovirus by using retrovirus system, then isolate T cells from the spleen of B6 mice, and finally infect mouse T cells with retrovirus, and the infection efficiency is detected by flow cytometry;
[0066] (2) Inject the prepared CAR-T cells into the irradiated B6 mice through the tail vein, 3 mice in each group, each injected with 5×10 5 CAR-T cells, the peripheral blood of the mice was regularly drawn to detect the growth of T cells and B cells ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com